Key takeaways:
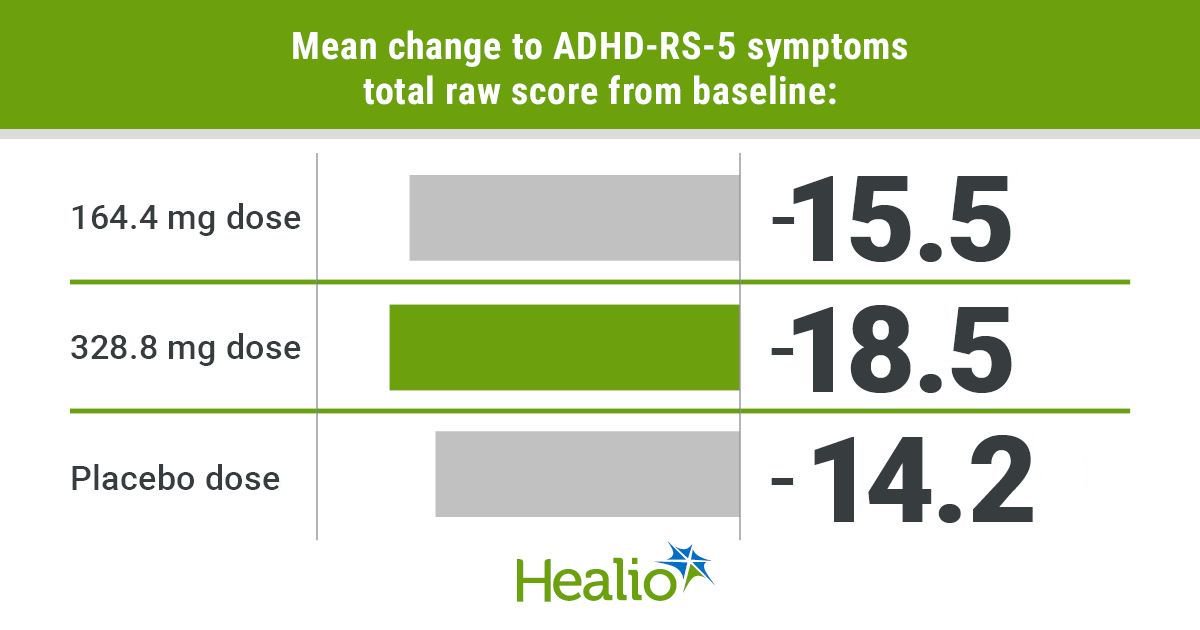
- The 328.8 mg dose demonstrated efficacy and speedy onset in contrast with placebo.
- Frequent opposed results, together with decreased urge for food, nausea, headache and rash, have been typically gentle to reasonable in severity.
A excessive dose of centanafadine was proven to be protected and efficient at reducing ADHD signs for adolescents, based on outcomes of a part 3 randomized trial printed in Journal of the American Academy of Baby & Adolescent Psychiatry.
Centanafadine (Otsuka Prescription drugs), a norepinephrine, dopamine, serotonin reuptake inhibitor, or NDSRI, has beforehand proven efficacy in two part 3 trials for adults with ADHD [published in 2022].

Knowledge have been derived from Ward CL, et al. J Am Acad Baby Adolesc Psychiatry. 2025;doi:10.1016/j.jaac.2025.06.023.
Thus, Timothy E. Wilens, MD, of Massachusetts Common Hospital in Boston, and colleagues determined to consider the remedy for adolescents in a double-blind, placebo-controlled trial.
The examine, carried out at 48 websites from 2022 to 2023, included 459 adolescents (imply age, 14.7 years, 59.3% male, 70.4% white) with a main analysis of ADHD. Researchers assigned the adolescents to one in every of three dosages, consisting of two capsules taken as soon as each day for six weeks:
- 164.4 mg dose: one 164.4 mg capsule, one placebo (n = 155);
- 328.8 mg dose: two 164.4 mg capsules (n = 155); or
- two placebo capsules (n = 149).
Adjustments within the ADHD Score Scale, model 5 (ADHD-RS-5) signs whole uncooked rating from baseline to week 6 served because the examine’s main endpoint, with adjustments on a modified Scientific World Impression of Severity to handle ADHD (CGI-S-ADHD) and T-scores for inattention, hyperactivity/impulsivity, defiance/aggression and government perform on the Conners 3-Dad or mum Brief content material scale, evaluated as secondary efficacy endpoints.
Sufferers had a imply baseline ADHD-RS-5 signs whole uncooked rating of 37.5 and a imply CGI-S-ADHD rating of 4.5.
Total, 80.8% of the individuals accomplished the examine, together with a safety-follow up interval.
Outcomes confirmed that adolescents assigned the 328.8 mg centanafadine dose had considerably improved signs as measured by adjustments from baseline to week 6 on ADHD-RS-5 in contrast with these assigned placebo (–18.5 vs. –14.2; P = .0006). These enhancements have been marked as early as the primary week and maintained all through the examine interval (P = .001).
Though individuals assigned the 164.4 mg dose additionally confirmed a discount in signs from baseline (–15.5), the distinction in contrast with the placebo group was not statistically vital.
The 2 centanafadine teams additionally confirmed larger enhancements on the CGI-S-ADHD in contrast with the placebo group, though once more solely the upper dose was considerably totally different (placebo, –1.06; 164.4 mg dose, –1.15; 328.8 mg dose, –1.42; P = .0038).
Moreover, the 328.8 mg dose group confirmed vital enhancements in contrast with the placebo group in inattention (imply distinction, –6.63; 95% CI, –9.06 to –3.6), hyperactivity/impulsivity (imply distinction, –5.52; 95% CI, –8.44 to –2.59) and government functioning (imply distinction, –4.92; 95% CI, –7.56 to –2.28) primarily based on week 6 Conners 3-Dad or mum Brief content material scale T-scores.
Therapy-emergent opposed occasions affected all three teams (164.4 mg dose, 31.4%; 328.8 mg dose, 50.3%; placebo, 23.8%), with the commonest signs being decreased urge for food, nausea, headache and rash.
Most opposed occasions have been thought-about gentle or reasonable, with all rash occasions resolved earlier than follow-up, based on the researchers. Just one participant reported suicidality deemed associated to the examine drug, which was thought-about gentle to reasonable in severity, they wrote.
Extreme occasions associated to centanafadine have been reported by three individuals, with two from the 164.4 mg group (liver perform take a look at elevated, aggression) that led to review discontinuation, and one from the placebo group (somnolence).
In keeping with the researchers, the outcomes present that the 328.8 mg dose specifically demonstrated security, efficacy and tolerability for adolescent ADHD remedy. Primarily based on the Conners 3-Dad or mum Rating knowledge, the upper dose can also be helpful at addressing ADHD-associated options, similar to government functioning, they added.
Though the 164.4 mg dose didn’t attain the first efficacy endpoint, the researchers point out the low dosage was nonetheless protected and well-tolerated.
They famous some examine limitations together with restricted generalizability to adolescents past North America, the exclusion of instructor scores on the ADHD-RS-5 scale and the examine’s brief period.
“The outcomes of this examine display the final tolerability and scientific advantages of centanafadine, an NDSRI, within the remedy of ADHD in an adolescent inhabitants,” the researchers wrote.
Future research ought to discover long-term remedy outcomes and efficacy in contrast with different ADHD therapies, in addition to its impact on treating ADHD with comorbid situations, they added.
Reference:
- Adler L, et al. J Clin Psychopharmacol. 2022;doi:10.1097/JCP.0000000000001575.