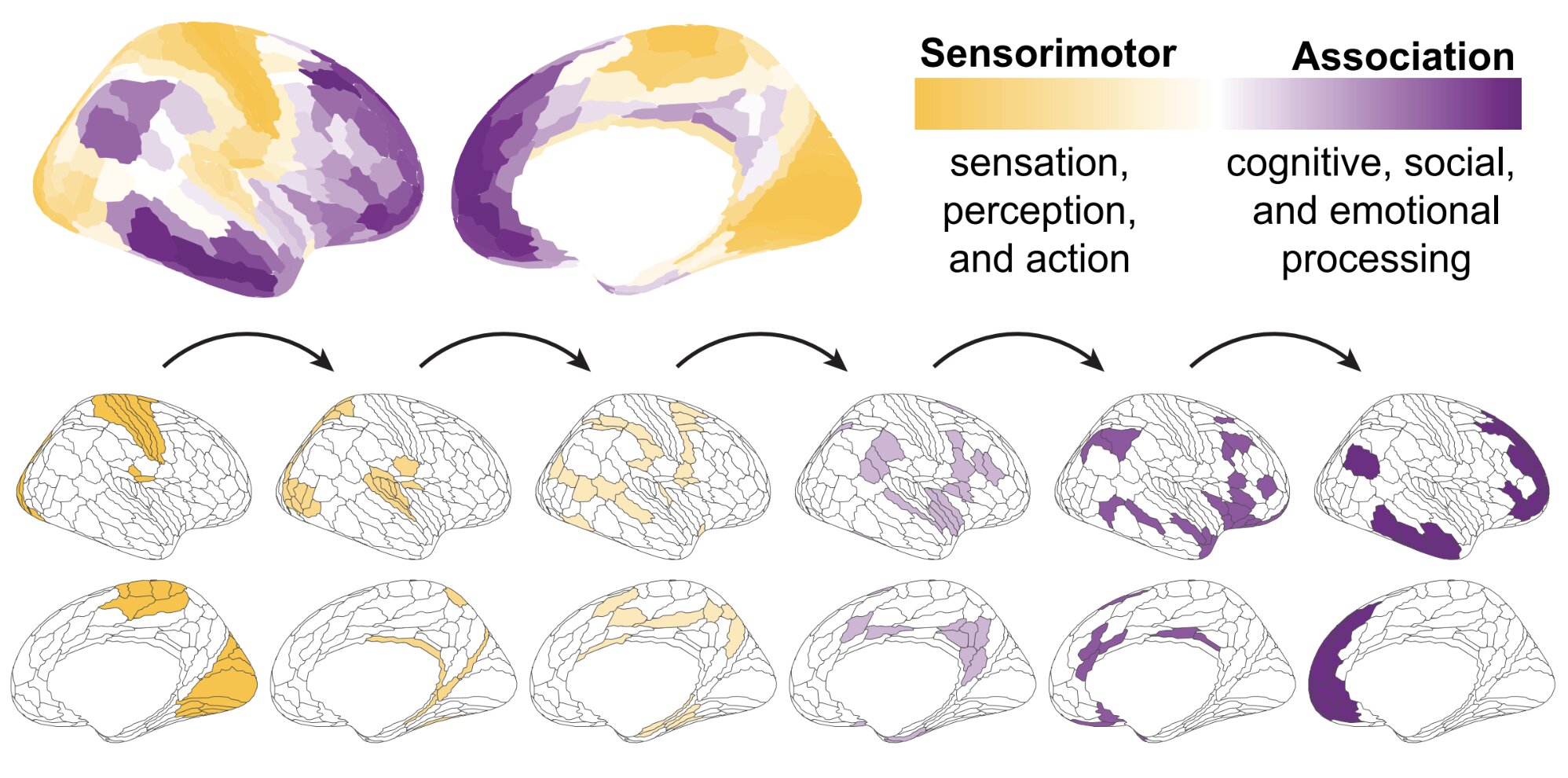
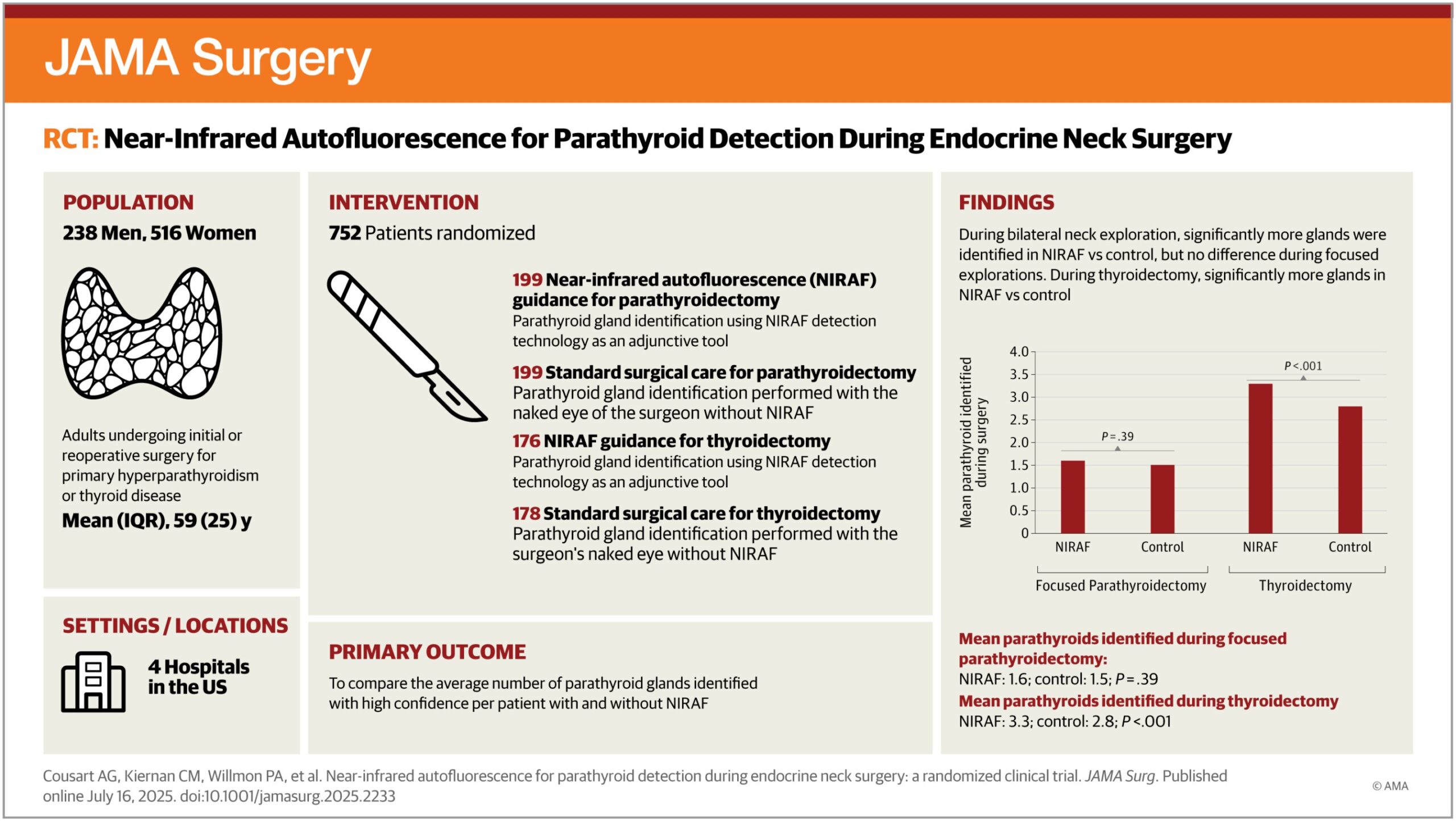
Key takeaways:
- Orforglipron lowered HbA1c and weight in contrast with placebo at 40 weeks for sufferers with kind 2 diabetes.
- Outcomes have been much like section 3 trials of semaglutide.
CHICAGO — Orforglipron, a novel nonpeptide, small molecule oral GLP-1, lowered HbA1c at 40 weeks for sufferers with kind 2 diabetes, in accordance with outcomes of the section 3 ACHIEVE-1 trial.
ACHIEVE-1 was offered on the American Diabetes Affiliation Scientific Classes and concurrently printed in The New England Journal of Medication. Julio Rosenstock, MD, FACE, senior scientific advisor for Velocity Medical Analysis, director of Velocity’s web site at Medical Metropolis Dallas and scientific professor of medication on the College of Texas Southwestern Medical Heart, Dallas, and colleagues randomly assigned 559 sufferers with kind 2 diabetes to once-daily orforglipron (Eli Lilly) 3 mg, orforglipron 12 mg, orforglipron 36 mg or placebo.

Present GLP-1s have been profitable, however “the hot button is, how can we get medicine which have much less uncomfortable side effects and have a extra sustained impact,” Rosenstock stated throughout a press convention. “There’s at all times room for innovation. This can be a small-molecule nonpeptide that has potential to open entry [to GLP-1 therapy] to extra individuals as a result of it’s simpler to take and is less complicated to supply, and in principle must be cheaper.”
At baseline, sufferers have been on no diabetes medicines, had HbA1c between 7% and 9.5% (imply, 8%) and had a BMI of a minimum of 23 kg/m2.
Reductions in HbA1c
At 40 weeks, imply change in HbA1c was –1.24 proportion factors within the orforglipron 3 mg group, –1.47 proportion factors within the orforglipron 12 mg group, –1.48 proportion factors within the orforglipron 36 mg group and –0.41 proportion factors within the placebo group, in accordance with the researchers.
The estimated imply distinction in HbA1c in contrast with placebo was 0.83 proportion factors (95% CI, 1.1 to 0.56) within the orforglipron 3 mg group, 1.06 proportion factors (95% CI, 1.33 to 0.79) within the orforglipron 12 mg group and 1.07 proportion factors (95% CI, 1.33 to 0.81) within the orforglipron 36 mg group (P < .001 for all), Rosenstock and colleagues discovered.
The efficacy estimand, which accounts for sufferers who took their remedy as meant, was related, which suggests the HbA1c outcomes are “extremely significant and really sturdy,” Rosenstock stated in the course of the press convention.
At 40 weeks, the imply HbA1c degree within the orforglipron teams ranged from 6.5% to six.7%, in accordance with the researchers.
Reductions in weight
Change in physique weight from baseline to 40 weeks was 4.5% within the orforglipron 3 mg group, 5.8% within the orforglipron 12 mg group, 7.6% within the orforglipron 36 mg group and 1.7% within the placebo group (P < .001 for orforglipron 12 mg and 30 mg vs. placebo), the researchers reported.
“The burden didn’t appear to succeed in a plateau,” Rosenstock stated in the course of the press convention. “It’s conceivable that we’d get additional discount [at later time points]. However for somebody with kind 2 diabetes, getting all the way down to regular [HbA1c] with 16 lb of weight reduction I believe is a heck of a response.”
There have been no instances of extreme hypoglycemia. The most typical hostile occasions have been gentle to average gastrointestinal occasions, most of which occurred throughout dose escalation, in accordance with the researchers. The speed of everlasting discontinuation of the research drug as a result of hostile occasions ranged from 4% to eight% within the orforglipron teams and was 1% within the placebo group.
Rosenstock stated the HbA1c and weight outcomes of ACHIEVE-1 have been much like these noticed within the PIONEER-1 trial of oral semaglutide (Rybelsus, Novo Nordisk) and the SUSTAIN-1 trial of injectable semaglutide (Ozempic, Novo Nordisk).
“This drug has the potential to be first-line remedy in sufferers with kind 2 diabetes,” Rosenstock stated in the course of the press convention.