Key takeaways:
- The addition of lurbinectedin to atezolizumab prolonged PFS and OS.
- The next proportion of sufferers assigned the mix skilled opposed occasions.
A novel fist-line upkeep remedy routine improved outcomes for sufferers with extensive-stage small cell lung most cancers, in response to examine outcomes scheduled for presentation at ASCO Annual Assembly.
The mix of lurbinectedin (Zepzelca; Jazz Prescription drugs, Pharma Mar), and atezolizumab (Tecentriq, Genentech) prolonged PFS and OS by a couple of months every in contrast with atezolizumab monotherapy, translating to a 46% decreased danger for development and a 27% decreased danger for loss of life.

Knowledge derived from Paz-Ares LG, et al. Summary #8006. Introduced at: ASCO Annual Assembly; Might 30-June 3, 2025; Chicago.

Hossein Borghaei
The findings of the randomized section 3 IMforte trial assist the mix as a possible new normal of care on this setting, researcher Hossein Borghaei, DO, MS, chief of thoracic medical oncology at Fox Chase Middle, mentioned in an interview.
“I might like to see a routine enhance survival by 6 months, 9 months or a yr,” Borghaei instructed Healio. “Nonetheless, in small cell lung most cancers, that has not been attainable. The outcomes are usually so poor and the necessity for brand new remedies is so nice, I believe we’ve to take our wins the place we will and hope to construct on them. So I might say this most likely does need to be the usual and, if this routine is obtainable, I might supply it to my sufferers.”
‘Devastating analysis’
Small cell lung most cancers — essentially the most aggressive subtype of the malignancy — accounts for roughly 15% of lung most cancers circumstances.
About 70% of small cell lung cancers are recognized as extensive-stage illness, which means it has unfold all through the lung or elsewhere within the physique, in response to examine background.
“It’s a devastating analysis,” Borghaei mentioned. “When the pathologist tells you it’s small cell, you wish to begin therapy instantly. There’s an actual sense of urgency as a result of it’s so aggressive, and since sufferers expertise so many signs.”
Normal therapy consists of induction remedy with etoposide, platinum chemotherapy and an immune checkpoint inhibitor — both atezolizumab (Tecentriq; Genentech) or durvalumab (Imfinzi, AstraZeneca) — adopted by upkeep remedy with the identical immune checkpoint inhibitor used throughout induction.
The introduction of immunotherapy has improved outcomes and plenty of sufferers with extensive-stage illness reply to induction remedy; nonetheless, a substantial portion of sufferers develop early relapse and prognosis stays poor. Median survival is 1 yr and solely 3% of sufferers survive 5 years.
Strategies
The IMforte trial enrolled 660 sufferers with treatment-naive extensive-stage small cell lung most cancers. All had ECOG efficiency standing of 0 or 1, with no historical past of mind or spinal wire metastases.
All trial contributors obtained normal induction remedy, which consisted of 4 cycles of the anti-PD-L1 antibody atezolizumab plus carboplatin and etoposide.
Sufferers who had ongoing tumor response or secure illness after induction remedy might proceed on examine.
Almost three-quarters (73.1%; n = 483) proceeded to upkeep remedy. That group included sufferers from 13 international locations (median age, 66 years; 63% males; 82% white, 13% Asian, 7% Hispanic or Latino).
Researchers randomly assigned 241 sufferers to upkeep with atezolizumab alone, dosed at 1,200 mg by way of IV each 3 weeks.
The opposite 242 obtained atezolizumab plus 3.2 mg/m2 lurbinectedin each 3 weeks. Lurbinectedin, an alkylating drug that binds to DNA and disrupts cell division, is authorized in america to deal with small cell lung most cancers that progressed after platinum-based chemotherapy.
Remedy continued till illness development or unacceptable toxicity, with no crossover allowed.
Unbiased evaluation facility-assessed PFS and OS served as major endpoints. Secondary endpoints included investigator-assessed PFS, general response price, length of response and security.
‘Clinically significant’ outcomes
Median follow-up was 15 months.
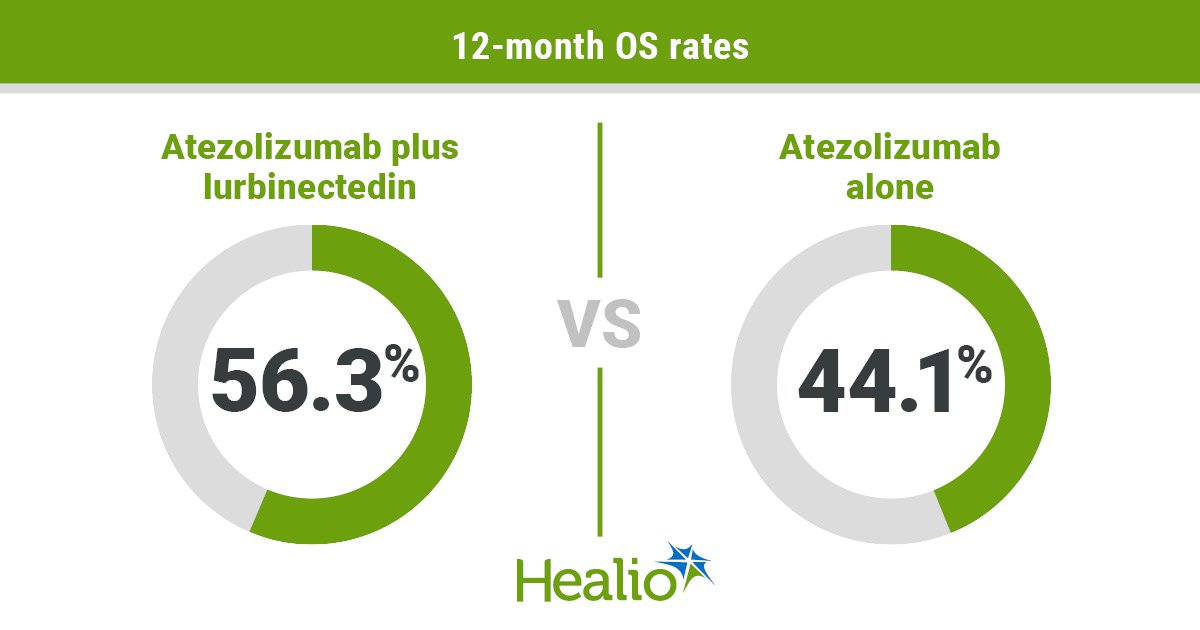
Outcomes confirmed statistically important enhancements in impartial evaluation facility-assessed median PFS (5.4 months vs. 2.1 months; HR = 0.54; 95% CI, 0.43-0.67), 12-month PFS price (20.5% vs. 12%), median OS (13.2 months vs. 10.6 months; HR = 0.73; 95% CI, 0.57-0.95) and 12-month OS price (56.3% vs. 44.1%) amongst sufferers assigned the mix.
Remedy length additionally favored the investigational arm. Sufferers assigned the mix remained on lurbinectedin for a median 4.1 months and on atezolizumab for a median 4.2 months. In distinction, these assigned to atezolizumab monotherapy remained on the agent for a median 2.1 months.
“For now, as a subject, I believe we’ve determined that 2 months or 3 months is clinically significant — particularly if a routine is mostly properly tolerated,” Borghaei mentioned. “Numerous research of medication we thought could be lively in small cell lung most cancers wound up failing so, when we’ve a constructive section 3 examine, it creates quite a lot of optimism. We begin to really feel like we’re bettering our understanding of the illness and a few of these newer therapy choices can assist us make progress.”
The next proportion of sufferers assigned the mix skilled any opposed occasion (97.1% vs. 80.8%), any grade 3/grade 4 opposed occasion (38% vs. 22.1%) and treatment-related grade 3/grade 4 opposed occasions (25.6% vs. 5.8%).
Opposed occasions that occurred extra steadily within the mixture group included nausea (36.4% vs. 4.2%), anemia (31.8% vs. 6.7%), fatigue (20.2% vs. 7.9%), decreased urge for food (16.9% vs. 6.7%), decreased platelet depend (15.3% vs. 2.9%), thrombocytopenia (12.8% vs. 1.7%), decreased neutrophil depend (12.8% vs. 1.3%) and neutropenia (10.7% vs. 1.7%).
The speed of grade 3/grade 4 infections appeared comparable (6.6% vs. 5%).
Twelve sufferers (5%) assigned the mix and 6 (2.5%) assigned atezolizumab alone skilled grade 5 opposed occasions, with two such occasions within the mixture group and one within the monotherapy group deemed therapy associated.
The next proportion assigned the mix discontinued any examine drug as a result of opposed occasions (6.2% vs. 3.3%) or had dose interruption/modification as a result of opposed occasions (38% vs. 13.8%).
“Toxicity must be an necessary a part of a therapy technique as a result of this affected person inhabitants sadly often has different comorbid situations, reminiscent of COPD, emphysema or maybe cardiac problems,” Borghaei mentioned. “The share of grade 3 and grade 4 toxicity highlights the necessity for continued shut follow-up of those sufferers till we change into extra comfy with this routine and learn the way these medication act. Each affected person is totally different, so the important thing can be to see these sufferers usually and ensure any toxicity that comes up is addressed shortly and successfully.”