Key takeaways:
- Kids with uncontrolled, average to extreme bronchial asthma used dupilumab or placebo for 52 weeks.
- The remedy group had important decreases in annualized exacerbation charges in contrast with the placebo group.
SAN FRANCISCO — Kids with bronchial asthma skilled fewer exacerbations and higher management with dupilumab whatever the length of their illness, based on a poster on the American Thoracic Society Worldwide Convention.
However kids with longer durations of bronchial asthma had extra exacerbations and fewer management, Wanda Phipatanakul, MD, MS, director, division of immunology analysis middle, Boston Kids’s Hospital, mentioned throughout her presentation.

Knowledge had been derived from Phipatanakul W, et al. Poster 8929. Offered at American Thoracic Scientific Congress; Might 16-21, 2005; San Francisco.
“There may be proof that elevated size of time with bronchial asthma can improve signs and result in persistent illness,” Phipatanakul advised Healio. “We wished to know whether or not illness length affected how dupilumab impacted key scientific outcomes.”

Wanda Phipatanakul
The 52-week, double-blind, part 3 VOYAGE trial included 350 kids aged 6 to 11 years with uncontrolled, average to extreme kind 2 inflammatory bronchial asthma, outlined as blood eosinophil counts of 150 cells/µL or greater or fractional exhaled nitric oxide of 20 components per billion or greater.
“The common affected person age was 9 years, and 64% had been male. Roughly 5% of sufferers had been Black/African American, 0.5% had been Asian/Asian American and 0.2% had been American Indian/Alaska Native,” Phipatanakul mentioned. “This inhabitants mirrored the demographic make-up of the research areas.”
Kids acquired dupilumab (Dupixent; Sanofi, Regeneron) in 100 mg or 200 mg doses relying on physique weight or placebo each 2 weeks in a 2:1 randomized ratio.
Cohorts included kids who had bronchial asthma for 4 years or fewer (37.7% of placebo group and 31.8% of dupilumab group), greater than 4 to fewer than 7 years (36.8% of placebo group and 36.9% of dupilumab group) or 7 years or extra (25.4% of placebo group and 31.4% of dupilumab group).
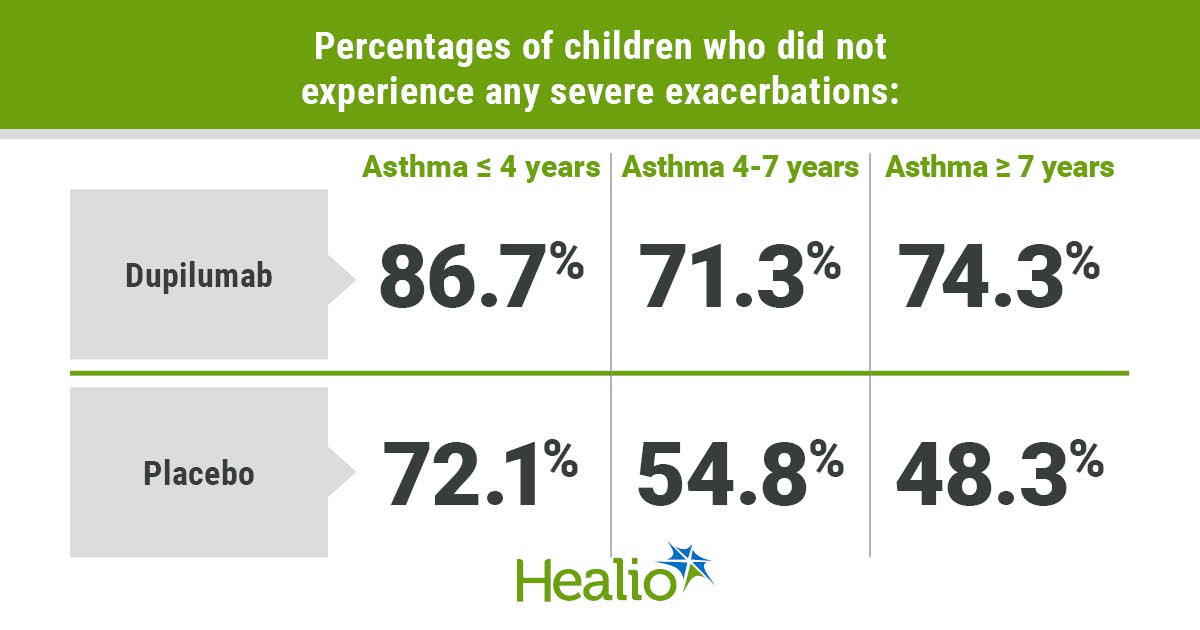
Percentages of kids who didn’t expertise any extreme exacerbations included 72.1% of these on placebo and 86.7% of these on dupilumab amongst these with bronchial asthma for 4 years or much less (P = .0439); 54.8% of these on placebo and 71.3% of these on dupilumab amongst these with bronchial asthma for greater than 4 to fewer than 7 years (P = .0359); and 48.3% of these on placebo and 74.3% of these on dupilumab amongst these with bronchial asthma for 7 years or longer (P = .0046).
In contrast with the placebo group, the dupilumab group additionally skilled important decreases in adjusted annualized exacerbation charges and enhancements in management, outlined as scores of 0.75 and decrease on the Interviewer-Administered 7-item Bronchial asthma Management Questionnaire.
Percentages of kids who reported well-controlled bronchial asthma included 55.8% of these on placebo and 78.7% of these on dupilumab amongst these with bronchial asthma for 4 years or much less (P = .011); 42.9% of these on placebo and 67.8% of these on dupilumab (P = .0104) amongst these with bronchial asthma for greater than 4 to fewer than 7 years; and 34.5% of these on placebo and 62.2% of these on dupilumab with bronchial asthma for 7 years or longer (P = .0189).
Phipatanakul referred to as the chances of exacerbation reductions with dupilumab in contrast with placebo in sufferers with bronchial asthma for 4 years or much less and sufferers with bronchial asthma for 7 years or longer older very comparable.
“Although the next proportion of sufferers with shorter illness durations skilled illness management and absence of extreme exacerbations total, nearly all of sufferers in every dupilumab group skilled these outcomes no matter how lengthy they’d had the illness and had been considerably extra possible than these on placebo to expertise them,” she mentioned.
Primarily based on these findings, the researchers concluded that dupilumab decreased annualized exacerbation charges and improved management amongst kids with uncontrolled average to extreme kind 2 bronchial asthma.
“The truth that dupilumab improves outcomes no matter earlier bronchial asthma length additional emphasizes its already well-established security and efficacy,” Phipatanakul mentioned.
“That is particularly essential for younger kids who’re significantly weak as their lungs are nonetheless creating, so lowering exacerbations and enhancing management may assist scale back the affect of persistent signs on the lungs,” she continued.
Phipatanakul famous that there are not any restrictions on the length of bronchial asthma earlier than dupilumab may be prescribed.
“However these information present physicians that whatever the length of their affected person’s illness, dupilumab can present clinically significant enhancements in indicators and signs in kids,” she mentioned.
For extra info:
Wanda Phipatanakul, MD, MS, may be reached at wanda.phipatanakul@childrens.harvard.edu.