Key takeaways:
- Tandem-target CAR-T produced a response in all individuals with relapsed or refractory mantle cell lymphoma in a section 1/2 examine.
- Sufferers acquired their CAR-T inside 8 to 12 days of lymphodepletion.
A quickly developed, dual-target chimeric antigen receptor T-cell remedy produced a 100% total response price in sufferers with relapsed or refractory mantle cell lymphoma in a section 1/2 examine.
In all, 88% of individuals achieved an entire response with CD20-CD19-directed remedy.

Moreover, each affected person acquired their remedy inside 8 to 12 days of beginning lymphodepletion.

Nirav N. Shah
“Twin concentrating on in mantle cell lymphoma, a illness characterised by brilliant CD20 expression, could have potential advantages based mostly on this scientific trial,” Nirav N. Shah, MD, MSHP, affiliate professor of drugs at Medical School of Wisconsin, advised Healio. “We’re excited to be taught extra by increasing on this single heart scientific trial with an ongoing multicenter trial that’s actively enrolling in [the trial].”
‘CD20 brilliant lymphoma’
Sufferers recognized with mantle cell lymphoma have a 5-year relative survival price of 55.6%, in accordance with knowledge from Metropolis of Hope.
Two CD19 CAR-T therapies have garnered approval for remedy — brexucabtagene autoleucel (Tecartus, Kite Pharma/Gilead Sciences) and lisocabtagene maraleucel (Breyanzi, Bristol Myers Squibb).
Nevertheless, roughly 15% of sufferers who obtain brexucabtagene autoleucel develop grade 3 or worse cytokine-release syndrome (CRS) and 31% develop grade 3 or worse immune effector cell-associated neurotoxicity syndrome (ICANS), in accordance with examine background.
Lisocabtagene maraleucel causes fewer opposed occasions, however sufferers have a decrease response price than brexucabtagene autoleucel and a PFS of solely 15.3 months.
“Biologically, mantle cell is a CD20 brilliant lymphoma, main us to hypothesize that concentrating on each CD19, which we all know is an efficient goal in CAR-T, and CD20, which is successfully harnessed with different kinds of medication, may result in higher outcomes,” Shah stated.
Healio beforehand reported that zamtocabtagene autoleucel (Miltenyi Biomedicine), additionally known as zamto-cel which is identical assemble as utilized in [the current] examine, produced a 72.8% total response price for sufferers with relapsed or refractory diffuse giant B-cell lymphoma within the DALY II USA Trial.
This trial carried out as a single heart examine centered solely on sufferers with mantle cell lymphoma who had failed two earlier strains of remedy or relapsed after transplant.
The trial included 17 sufferers (median age, 63 years; vary 50-74; 88% males; median strains of prior remedy, 4; vary, two-eight). The section I security portion of the trial included three sufferers, and the section 2 single-arm part had 14.
Sufferers started lymphodepletion after which acquired one dose of 20.19 CAR-T cells (a product with the identical assemble as zamto-cel and on the identical dose 2.5 x 106 CAR T cells/kg as soon as produced). Researchers reported a producing strategy of 8 to 12 days.
The first endpoint was the 3-month CR price. Total response price, full response price, minimal residual illness, length of remission, security outcomes, PFS and OS served as secondary endpoints.
‘We had been happy’
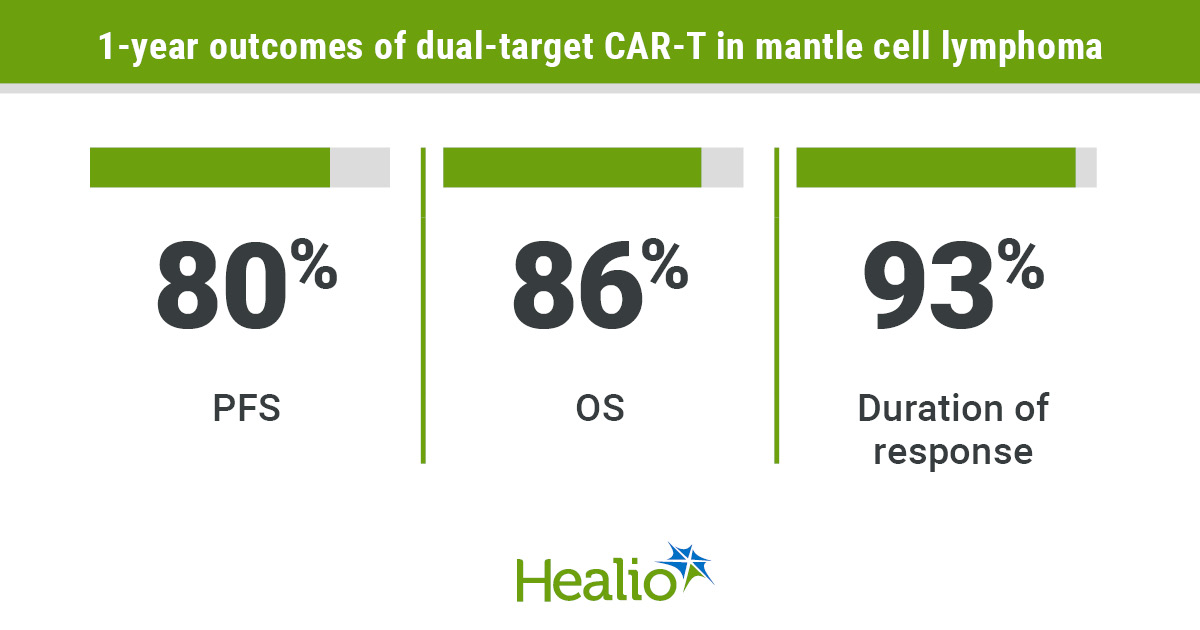
Individuals had an ORR of 100%, with a finest full response price of 88%.
Sufferers within the section 2 cohort had an entire response price of 86% on day 90.
Total, two sufferers relapsed, one at 8 months and one other at 24 months.
At median follow-up of 15.8 months, median PFS, length of response and OS didn’t get reached.
Individuals had a 1-year PFS of 80% (95% CI, 49%-93%), 1-year length of response of 93% (95% CI, 61%-99%) and 1-year OS of 86% (95% CI, 55%-96%).
Of 14 evaluable sufferers, 79% had no minimal residual illness at median follow-up of 96 days (vary, 85-258).
“We had been happy,” Shah stated.
Almost all individuals (94%) developed grade 1 or 2 CRS. None developed grade 3 or worse illness.
Any-grade ICANS occurred in 18% of sufferers, and grade 3 occasions developed in 12%. Clinicians resolved the grade 3 ICANS.
“I’d say that the toxicity profile of that is much like lisocabtagene maraleucel,” Shah stated.
In all, three sufferers died of non-relapse occasions associated to infectious problems.
‘Any malignancy in lymphoma’
A multicenter, section 2 investigation is ongoing.
Researchers need to accrue roughly 70 sufferers with mantle cell lymphoma inside a 12 months as a part of this multicenter examine, Shah stated.
“We nonetheless don’t have a great understanding as to why CAR-T was so profitable in some, and why others relapsed,” Shah stated. “The opposite large factor is [that] this seems to be good in a single-center examine. We’re very skilled with this CAR-T and so you will need to consider in a multicenter style, which we have now initiated as a part of the DALY II USA scientific trial.. How will this CAR-T develop and evolve as we disperse it?”
Future analysis could contain first-line trials and analysis in different ailments.
“We’ve beforehand offered knowledge in power lymphocytic leukemia,” Shah stated. “We’ve had some very nice outcomes, however that knowledge are nonetheless maturing. By concentrating on CD20 and CD19, you actually open your self as much as nearly any malignancy in lymphoma. CD20 is probably the most ubiquitous goal on the market in lymphoma. You see it in marginal zone lymphoma, follicular lymphoma, CLL, mantle cell and diffuse giant B cell lymphoma.
“We centered initially on DLBCL and mantle as a result of these are ones the place there’s a transparent scientific indication for CAR-T, however we hope to proceed to develop this for different B cell malignancies, as properly.”
References:
For extra info:
Nirav N. Shah, MD, MSHP, might be reached at nishah@mcw.edu.