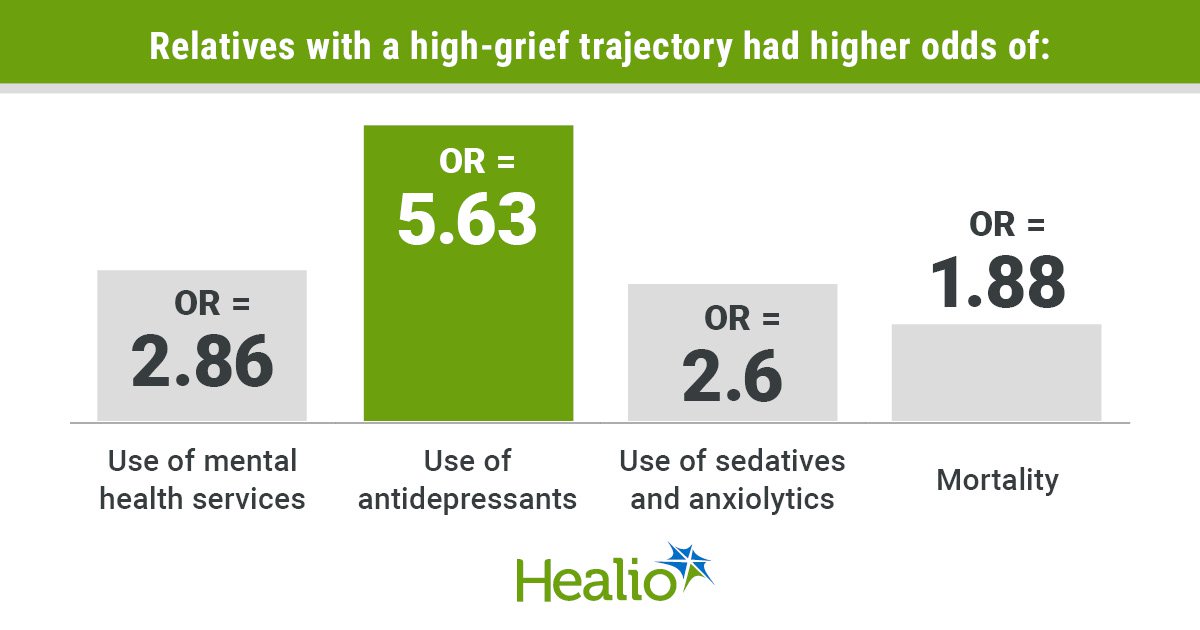
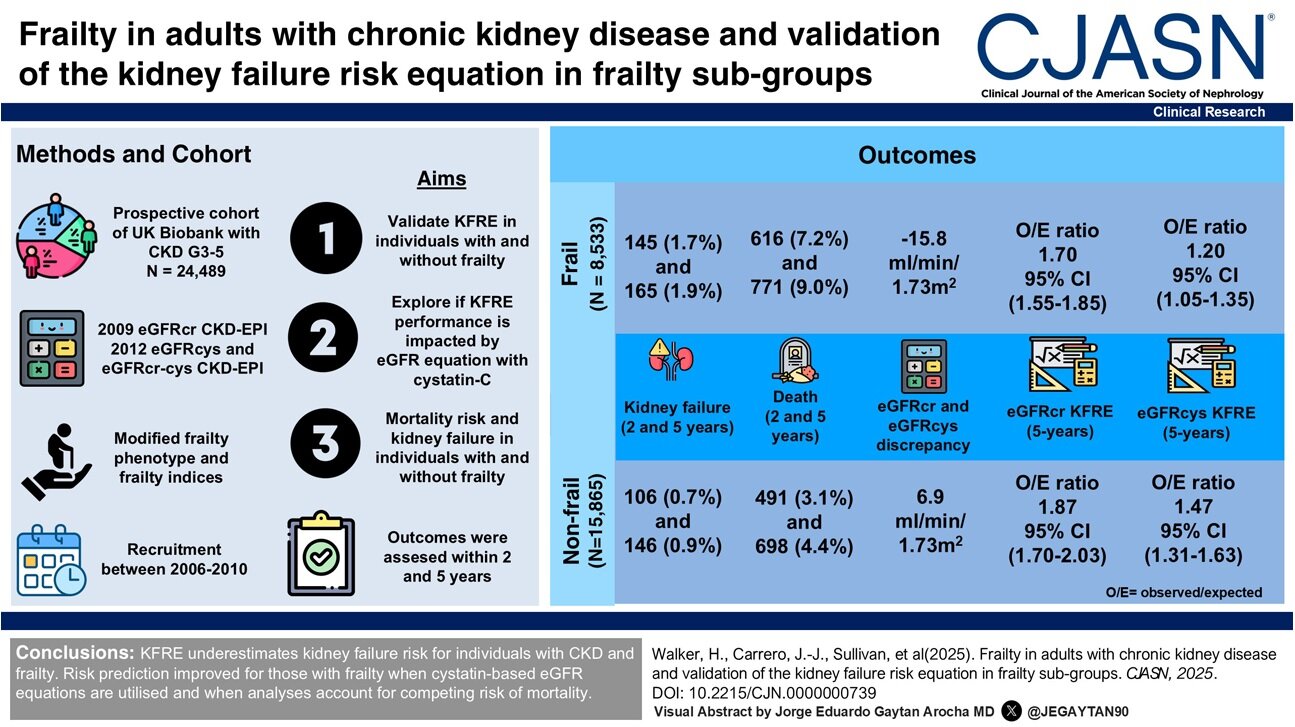
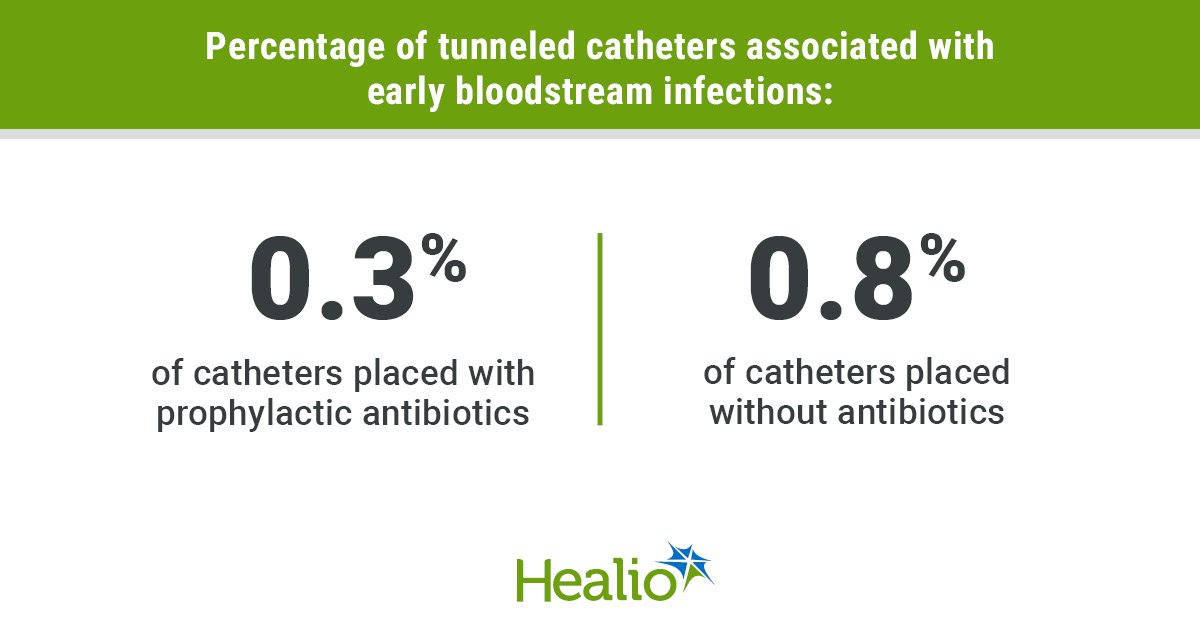
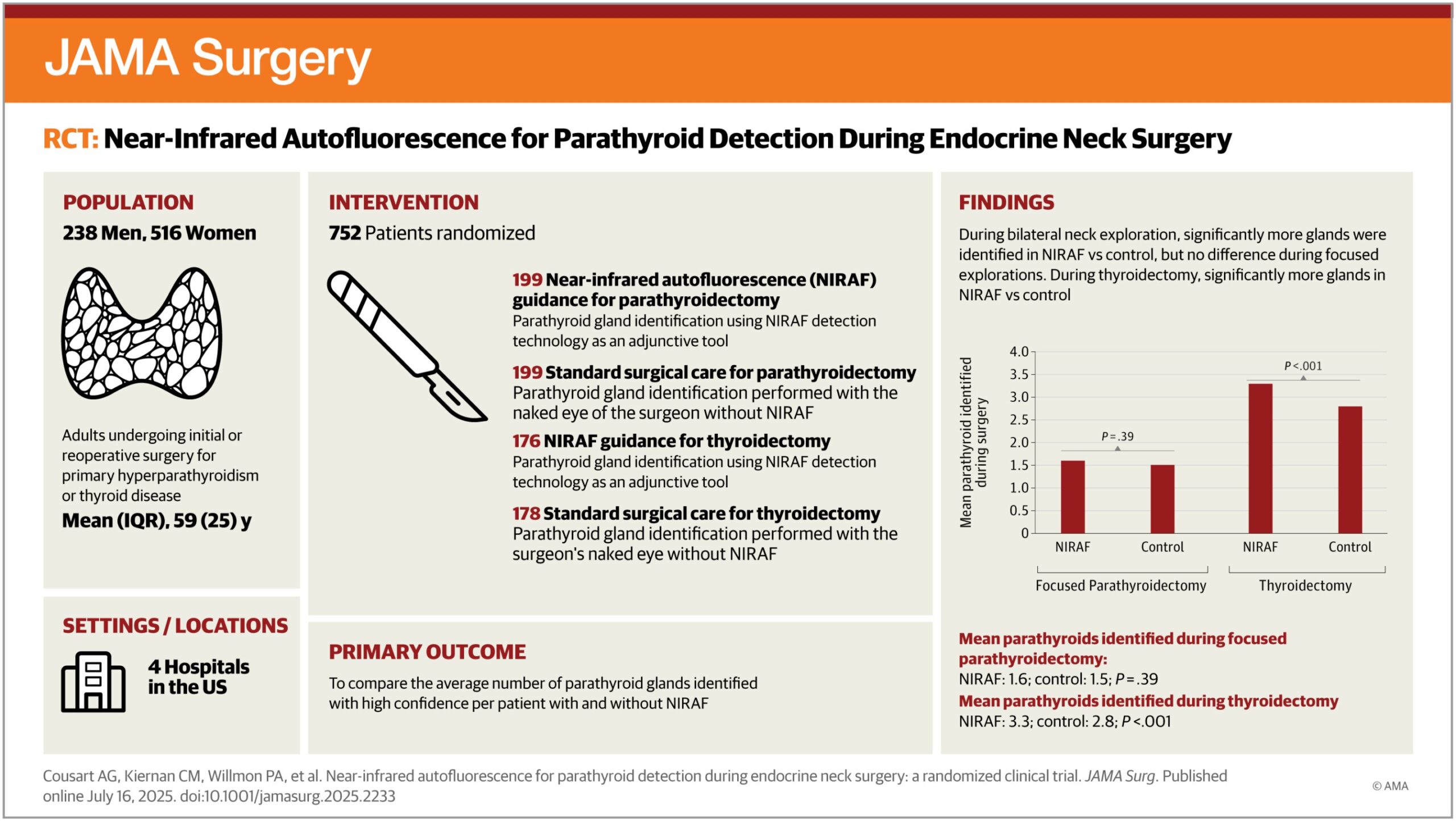
Key takeaways:
- The designation was supported by to-date outcomes of the continuing SPECTRA examine in diabetic macular edema.
- The gene remedy is designed to supply sustained supply of anti-VEGF after one injection.
The FDA granted regenerative medication superior remedy designation to 4D-150 for the remedy of diabetic macular edema, in line with a press launch from 4D Molecular Therapeutics.
The gene remedy is designed to supply sustained supply of anti-VEGF (aflibercept and anti-VEGF-C) with a single intravitreal injection. The regenerative medication superior remedy (RMAT) designation is granted with the intent of expediting the event and assessment of therapies, providing sponsor firms the advantages of quick monitor and breakthrough remedy designations, the discharge mentioned.

The designation was supported by to-date outcomes of the continuing SPECTRA examine in DME, in line with David Kirn, MD, co-founder and CEO of 4DMT.
“This designation in DME follows the RMAT designation granted for 4D-150 in moist AMD, and to our data, 4D-150 is the primary investigational medication to be granted the designation in each indications,” he mentioned. “We sit up for persevering with our ongoing collaboration with the FDA to advance 4D-150 into part 3 growth with an aligned-upon single part 3 trial for approval in DME, mixed with our two moist AMD 4FRONT part 3 medical trials.”