Key takeaways:
- The gadget considerably improved high quality of life and signs amongst ladies with urgency urinary incontinence over 24 months.
- Members who didn’t obtain the trial’s efficacy endpoint nonetheless benefitted.
An implantable tibial neuromodulation remedy considerably improved high quality of life amongst ladies with urgency urinary incontinence, based on a examine introduced on the American Urological Affiliation Annual Assembly.
Additionally printed in The Journal of Urology, the examine confirmed the advantages related to the novel implantable tibial neuromodulation system, generally known as Revi, have been sustained all through 24 months in ladies with urgency urinary incontinence (UUI).

Knowledge derived from: Dmochowski R, et al. High quality-of-life outcomes of implantable tibial neuromodulation for urgency urinary incontinence: two-year outcomes from the OASIS pivotal examine utilizing the Revi System. Introduced at: American Urological Affiliation Annual Assembly; April 26-29, 2025; Las Vegas (hybrid assembly).
“Revi offers a extremely efficient, sustainable and well-tolerated remedy possibility for sufferers with UUI who’re dwelling with disruptions to their high quality of life,” Roger R. Dmochowski, MD, MMHC, a professor of urology and surgical procedure at Vanderbilt College Medical Heart and chief medical officer at BlueWind Medical, instructed Healio.
Revi is “a small gadget implanted close to the tibial nerve within the ankle in a single outpatient process,” Dmochowski stated. “Sufferers put on an exterior, battery-operated gadget that prompts the implant for roughly half-hour per day, stimulating the nerve to assist management bladder operate.”
Based on the Cleveland Clinic, individuals with UUI have a sudden urge to urinate — typically leading to unintended leakage — that happens a number of occasions through the day and night time.
Within the Overactive Bladder Stimulation System Examine (OASIS), the efficacy and security of Revi was assessed amongst 151 ladies with UUI by voiding diaries and quality-of-life questionnaires, the latter of which have been collected at 6, 12 and 24 months after the gadget was implanted.
The remedy’s response charge — the trial’s efficacy endpoint, which was outlined as a 50% or better lower in UUI episodes — was 77.8% at 6 months, 82% at 9 months and 79.4% at 24 months.
The researchers discovered that Revi was tied to vital and sustained enhancements in all of the domains of the Overactive Bladder Questionnaire, together with coping, sleep, social and concern.
There was an almost 40-point discount in symptom severity rating through the examine, reducing from 70.3 at baseline to 31.5 at 24 months.
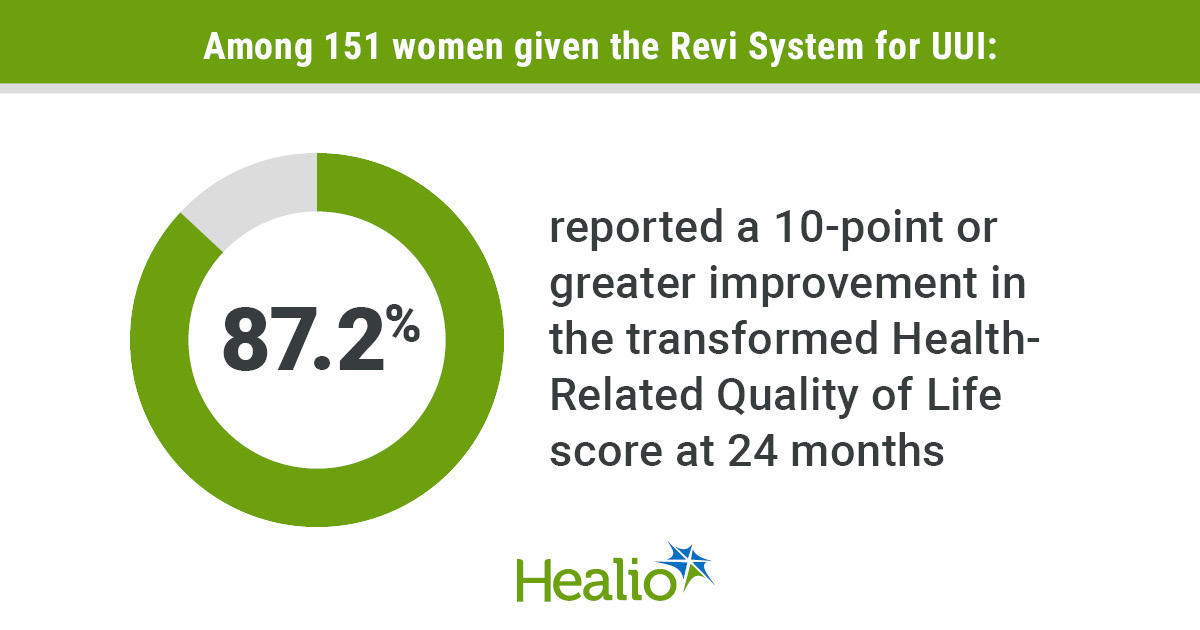
In the meantime, 87.2% of individuals reported a 10-point or better enchancment within the whole reworked Well being-Associated High quality of Life rating at 24 months.
At that time limit, most individuals reported remedy profit (96.8%) and remedy satisfaction (96.7%).
Dmochowski and colleagues reported that the 21% of sufferers who didn’t meet the efficacy endpoint at 24 months nonetheless confirmed appreciation of Revi, with:
- 83.3% acknowledging remedy profit;
- 66.7% reporting remedy satisfaction; and
- 100% reporting a willingness to proceed.
Dmochowski stated that Revi “is particularly suited to the massive ‘watchful ready’ inhabitants — these dwelling with untreated or inadequately managed UUI.”
“It provides a minimally invasive, extremely customizable answer with out an implanted battery,” he stated.
Dmochowski concluded that the gadget “permits early intervention with out requiring step-therapy failures and provides a non-drug, sturdy answer that considerably improves high quality of life throughout crucial domains like sleep, social operate and emotional well-being.”
References:
For extra info:
Roger R. Dmochowski, MD, MMHC, might be reached at primarycare@helio.com.