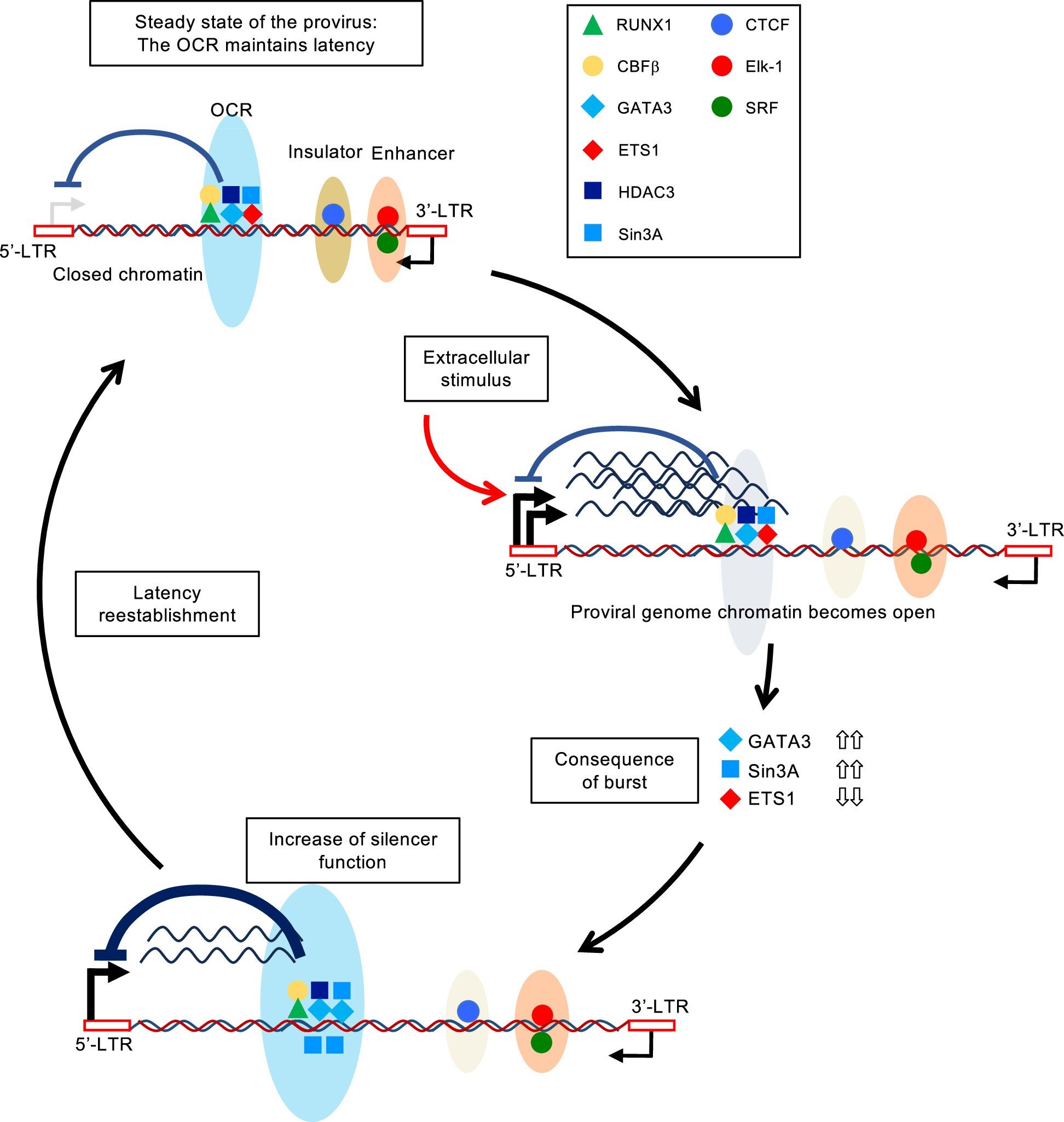
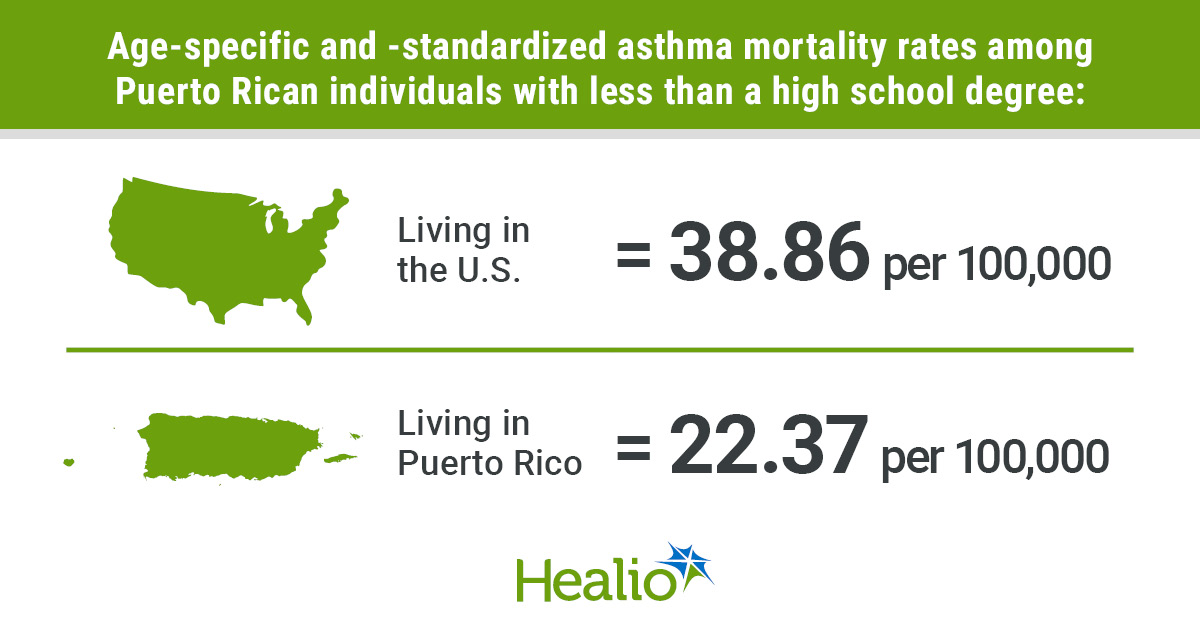
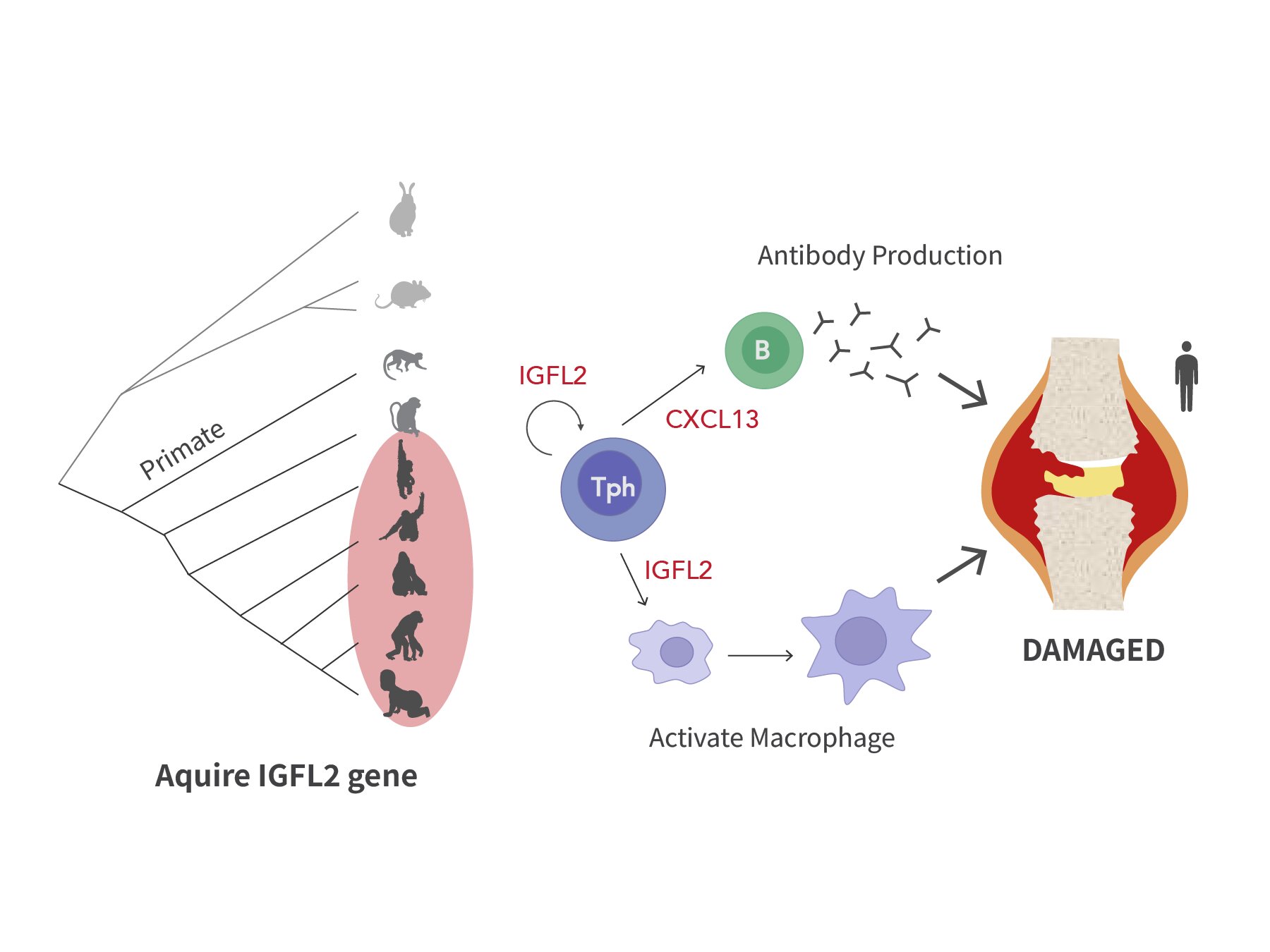
Need to keep on prime of the science and politics driving biotech in the present day? Join to get our biotech publication in your inbox.
Good morning, let’s get into all of the updates from a busy earnings season to date.
The necessity-to-know this morning
- GSK and United Therapeutics reported earnings.
- Novartis is buying Regulus Therapeutics, developer of an experimental therapy for ADPKD, an inherited sort of kidney illness, for $800 million upfront plus one other $900 million contingent on the drug’s approval.
BridgeBio delivers a giant beat with new coronary heart drug
BridgeBio’s not too long ago accepted coronary heart drug introduced in $36.7 million in gross sales within the first quarter, blowing previous expectations. (Analysts had estimated $12 million, however Wall Avenue’s “whisper quantity” was $17 million to $20 million.)
The brand new numbers present that the biotech is off to a promising begin with its drug, referred to as Attruby, launched final November. The corporate is changing into a extra formidable participant within the house simply as Alnylam begins to launch its competing drug, which was accepted final month.
For years, Pfizer was the one drugmaker with therapies for the illness, referred to as ATTR-CM, however BridgeBio’s CEO stated in an interview that there’s room for different merchandise as extra docs turn out to be conscious of the illness and sufferers get recognized earlier.
Pfizer CEO thinks pharma tariffs gained’t be utilized equally
From my colleague Adam Feuerstein: On the pharma big’s earnings convention name yesterday, Pfizer CEO Albert Bourla supplied a noteworthy tackle the opportunity of pharmaceutical tariffs.
The Trump administration is motivated at the very least partially by nationwide safety considerations, Bourla stated, which may result in completely different tariff charges for international locations deeemed “pleasant” or “adversary.”
“I believe no nation needs to have crucial medicines produced exterior of the nation, notably, they don’t wish to have crucial medicines produced in international locations the place tensions are excessive or can escalate,” stated Bourla.
He added that China’s quickly advancing biotech trade is also seen as a nationwide safety risk:
“The factor that I talk about with everybody within the administration, and I believe that resonates rather a lot with Democrats and with Republicans, with all people within the administration, together with HHS and in all places, is that China proper now’s progressing with the pace of sunshine of their science. Biotech, it’s dominant.
The U.S. has the dominant scientific place in biotech as a result of now we have created this ecosystem of biotech and pharma and academia and all of that. That is precisely what the Chinese language have replicated within the final 4 years. And within the final 4 years, there was a big setback [with IRA] for the U.S. that took down lots of funding for personal sector biotechs.”
A tariff scheme that largely spares Eire however punishes China can be a win for pharma, stated Evercore ISI analyst Umer Raffat, in a word to traders.
The following huge vaccine developments to observe
Within the brief time that RFK Jr. has been HHS secretary, he’s already proven that that the intends to make use of his intensive powers over regulation, coverage, and analysis funding to affect Individuals’ interested by vaccines.
However over the following couple of months, he’ll can have many extra alternatives to pursue his decades-long marketing campaign in opposition to vaccines. My colleague Helen Branswell compiled an inventory of the important thing developments to observe.
Along with scrutinizing how the FDA handles its overview of Novavax’s Covid vaccine and Moderna’s next-generation Covid vaccine, she’s additionally how well being companies will reply to advisory committees, in addition to the destiny of presidency contracts with vaccine makers.
A brand new smoking cessation drug goes earlier than FDA
Obtain Life Sciences will quickly file for approval of its drug, referred to as cytisinicline, that goals to assist individuals fairly smoking. If cleared by regulators, it might be the primary new drug for smoking cessation in 20 years.
There are presently solely two drugs in the marketplace to assist individuals quit smoking — the more practical of which may include disagreeable unwanted effects like nausea that make individuals much less prone to follow the therapy.
Pharma corporations have shied away from creating new smoking cessation medication, partially as a result of the FDA has traditionally taken a cautious strategy to reviewing that sort of medicine.
My colleague Sarah Todd sat down with Obtain’s CEO to speak in regards to the information it’s collected and the corporate’s technique for getting approval. Learn extra.
Extra reads
- Q&A: In dialog with FDA Commissioner Dr. Marty Makary, Inside Drugs
- Well being methods in limbo as HHS stays quiet on nondiscrimination guidelines for AI, algorithms, STAT
- Opinion: A harmful new push to ban embryonic stem cell analysis funding is gaining momentum, STAT