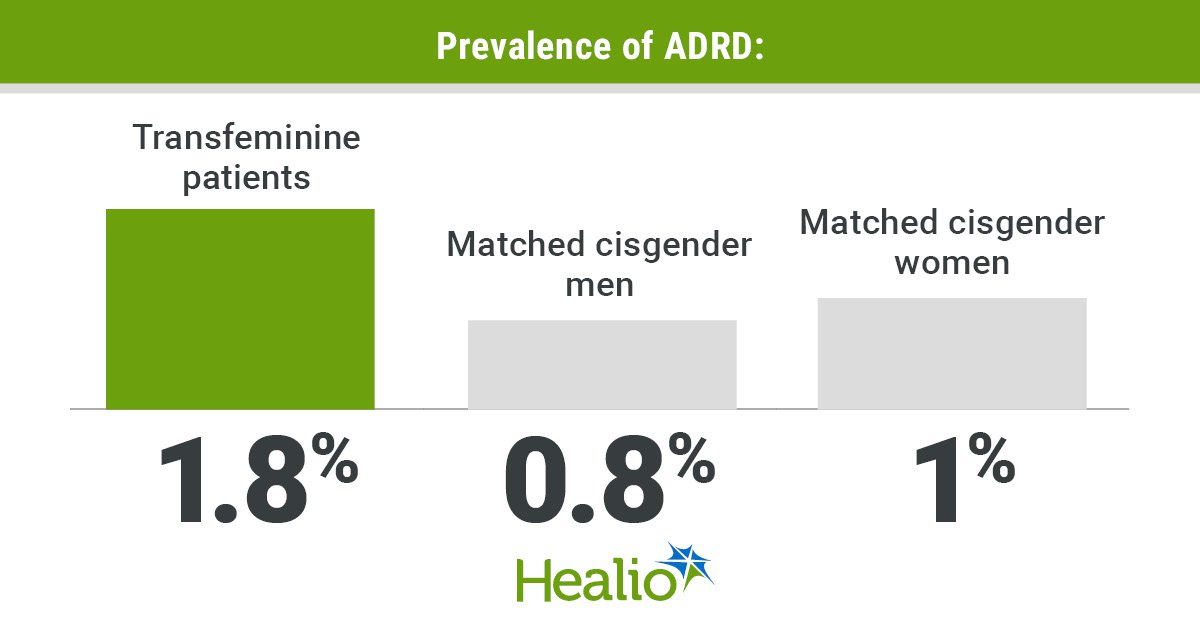
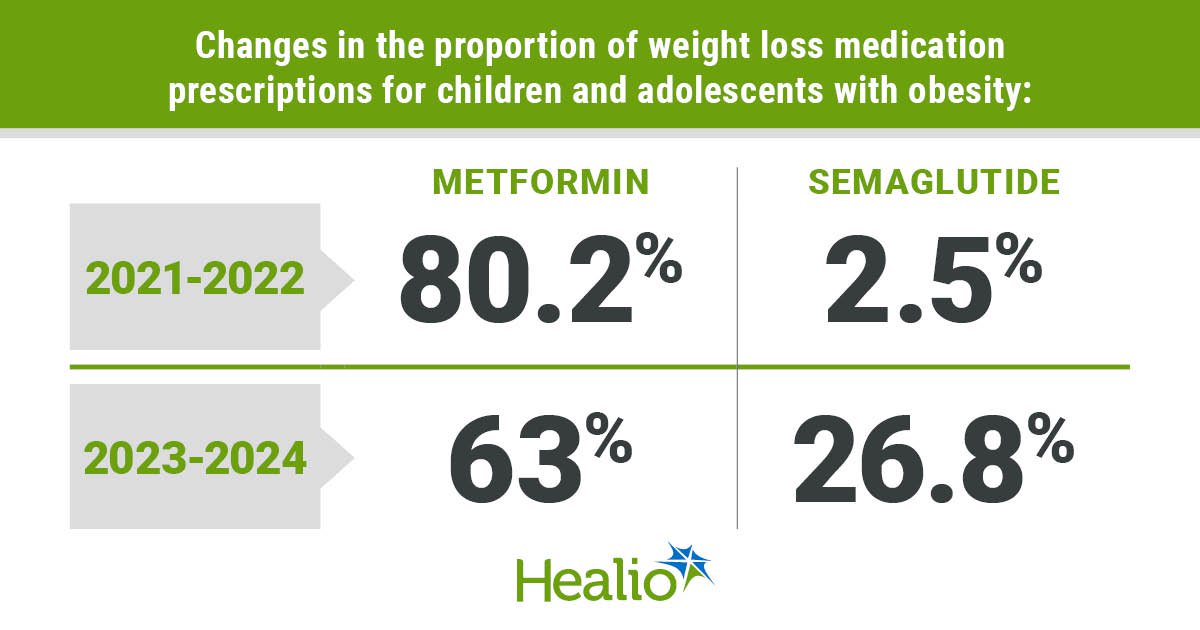
Prime of the morning to you. And a superb one it’s. A lot of sunshine and clear blue skies are as soon as once more enveloping the Pharmalot campus, the place the official mascots are bounding concerning the grounds in quest of creatures to terrorize. As for us, we’re as busy as ever looking and gathering gadgets of curiosity. We belief you might have your personal hectic agendas. So be part of us as we hoist the ever-present cup of stimulation — our selection at the moment is roasted coconut — and assault the fast-growing to-do listing. Have a grand day, everybody, and do keep in contact. We eagerly welcome suggestions, suggestions, criticism, and recent concepts. …
Merck will develop its U.S. manufacturing footprint with a $1 billion plant in Delaware, changing into the most recent drugmaker to spend money on the U.S. as tariffs focusing on the business loom, The Wall Road Journal stories. The ability will produce varied merchandise, together with biologic medicine and a brand new, easier-to-use model of Keytruda, the corporate’s blockbuster most cancers drug. The plant will develop into Merck’s first in-house manufacturing web site within the U.S. to make Keytruda, and can guarantee American sufferers get the drug made domestically. Keytruda accounts for roughly half of the corporate’s income, and final 12 months generated $29.5 billion in worldwide gross sales. The drug is manufactured outdoors the U.S. in locations reminiscent of Eire and by home contract producers. Merck has stated it has constructed up sufficient U.S. provide for 2025 and is engaged on increasing manufacturing for the long run. The corporate just lately projected tariffs carried out to this point will price the corporate $200 million.
File this below ‘So shut, but to this point.’ After a number of years of battling regulatory hurdles to win approval for its uncommon illness drug, Stealth BioTherapeutics had anticipated the U.S. Meals and Drug Administration to reply on Tuesday to its advertising software, STAT tells us. However late final week, the corporate obtained a letter saying there was a delay. Furthermore, the company didn’t point out when it could now full its evaluation for the drug, which known as elamipretide and was developed to fight Barth syndrome. The uncommon sickness, which causes an enlarged coronary heart, muscle weak spot, and a shortened life expectancy, afflicts practically 150 individuals within the U.S. An FDA spokesperson declined to touch upon the explanations for the delay in assembly the official date, citing confidentiality, and referred us to the corporate. The sequence of occasions, nonetheless, signifies the delay might be traced to upheaval on the FDA, based on a former FDA official accustomed to the matter.


This text is unique to STAT+ subscribers
Unlock this text — plus in-depth evaluation, newsletters, premium occasions, and information alerts.
Have already got an account? Log in