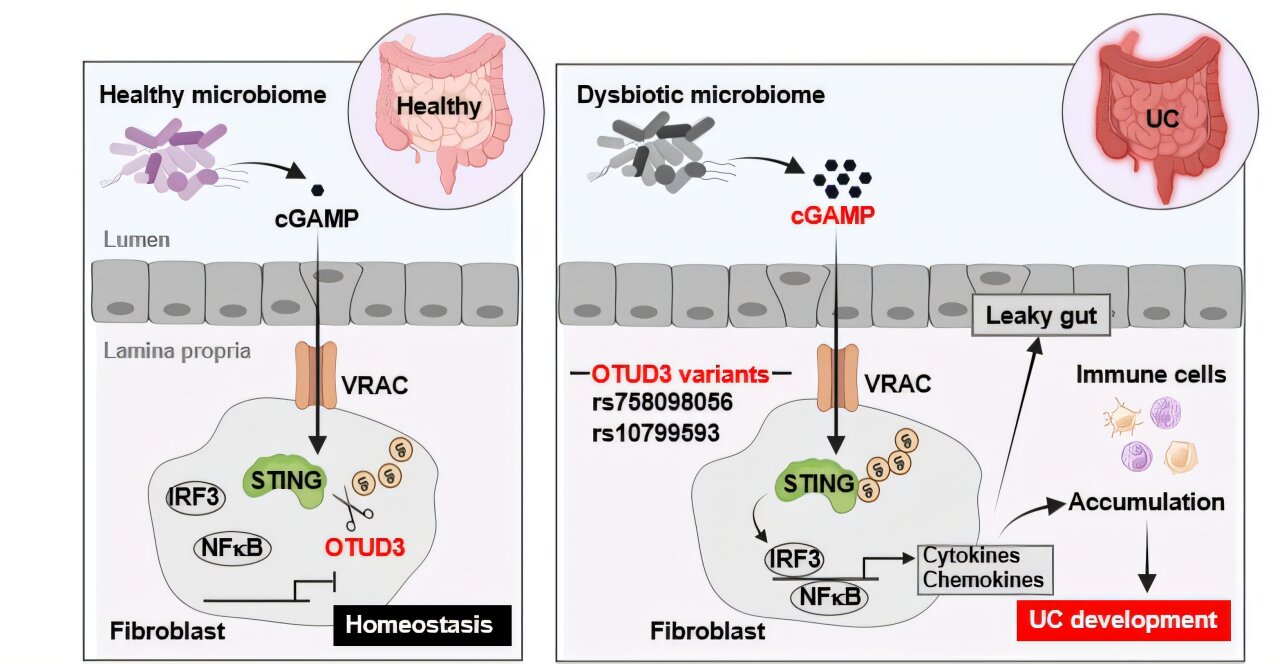
And so, one other working week will quickly draw to a detailed. Not a second too quickly, sure? That is, you could recall, our treasured sign to daydream about weekend plans. Our agenda, to this point, stays considerably unsettled. Apart from escorting Mrs. Pharmalot to an occasion, we plan to compensate for our studying, promenade with the official mascots, and manicure the grounds. We additionally hope to carry one other listening get together, the place the rotation will possible embody this, this, this, this and this. And what about you? This can be a chance to take pleasure in some sunshine if temperatures allow. Or if the warmth is on, you can curl up in entrance of the telly for a bit. Maybe this is a chance to ramble about city and pattern attention-grabbing meals. Or just plan the remainder of your life. Nicely, no matter you do, have a grand time. However be secure. Take pleasure in, and see you in later this month. Sure, we’re taking a break and some pinch hitters will substitute for us in our absence. Ta-ta, everybody. …
In a shock, a U.S. Meals and Drug Administration advisory panel voted that dangers tied to a GSK blood most cancers drug outweighed the advantages demonstrated in trials, as considerations about generally severe eye-related negative effects and questions in regards to the dose the corporate chosen dominated a listening to, STAT tells us. The FDA is about to determine whether or not to approve the drug, known as Blenrep, as quickly as subsequent week. GSK was attempting to revive the drug, which was pulled from the U.S. market in 2022 after failing a late-stage examine. In briefing paperwork launched earlier this week, FDA employees highlighted their considerations in regards to the ocular points and steered decrease dosages might provide sufferers related efficacy in treating a number of myeloma whereas reducing the chance of negative effects. In the course of the Oncologic Medication Advisory Committee assembly, GSK officers mentioned they chose dosages that supplied most advantages for sufferers and submitted two totally different Blenrep combos to the FDA as a number of myeloma remedies for sufferers who had not less than one prior line of remedy.
A 51-year-old man died final month after receiving an experimental gene remedy developed by Sarepta Therapeutics for an ultra-rare type of muscular dystrophy, STAT writes. He’s the third affected person to die after getting a Sarepta gene remedy this 12 months, all tied to liver points. Two teenage boys died after receiving Elevidys, Sarepta’s gene remedy for Duchenne muscular dystrophy, forcing the corporate to take the drug off the marketplace for older sufferers. The newest demise occurred in a scientific trial for limb girdle muscular dystrophy, a set of rarer and usually slower-moving situations. The demise will possible additional stoke security considerations round Sarepta’s gene therapies and lift questions across the firm’s transparency. Sarepta didn’t disclose the affected person demise on Wednesday, when it introduced a restructuring and shedding 500 staff. The corporate revealed it was discontinuing all however one in all its limb girdle packages — it was creating remedies for six totally different subtypes — however described it as a financially motivated choice, as all subtypes are fairly uncommon.


This text is unique to STAT+ subscribers
Unlock this text — plus in-depth evaluation, newsletters, premium occasions, and information alerts.
Have already got an account? Log in