Teclistamab-cqyv is a T-cell-engaging bispecific antibody that targets a number of myeloma cells through the B-cell maturation antigen (BCMA) receptor. It acquired accelerated approval in 2022 for sufferers handled with 4 or extra traces of prior remedy based mostly on outcomes from the Section I/II MajesTEC-1 medical trial.
Nonetheless, the potential advantages of the bispecific immunotherapy in populations not represented within the trial, or within the presence of threat components related to poorer outcomes, stays an necessary focus of ongoing medical investigation.
“Teclistamab is a crucial remedy possibility for sufferers with relapsed/refractory a number of myeloma however there may be nonetheless quite a bit to find out about easy methods to modify threat components and optimize using teclistamab in medical follow,” stated Beatrice M. Razzo, MD, an assistant professor at Thomas Jefferson College, former hematology-oncology fellow on the College of Pennsylvania, and the lead writer of the real-world research involving sufferers handled in a consortium of 15 U.S. educational medical facilities.
Within the largest research of its form to this point, Razzo and her staff retrospectively analyzed knowledge from 509 a number of myeloma sufferers, half of whom had acquired at the least six prior remedies.
The findings have been revealed in Blood Most cancers Discovery.
Some 89% (453) of those sufferers would have been ineligible for MajesTEC-1, with the most typical causes being prior remedy with one other BCMA-targeting remedy (236 sufferers), cytopenias (189 sufferers), and an ECOG efficiency standing of two or greater (117 sufferers).
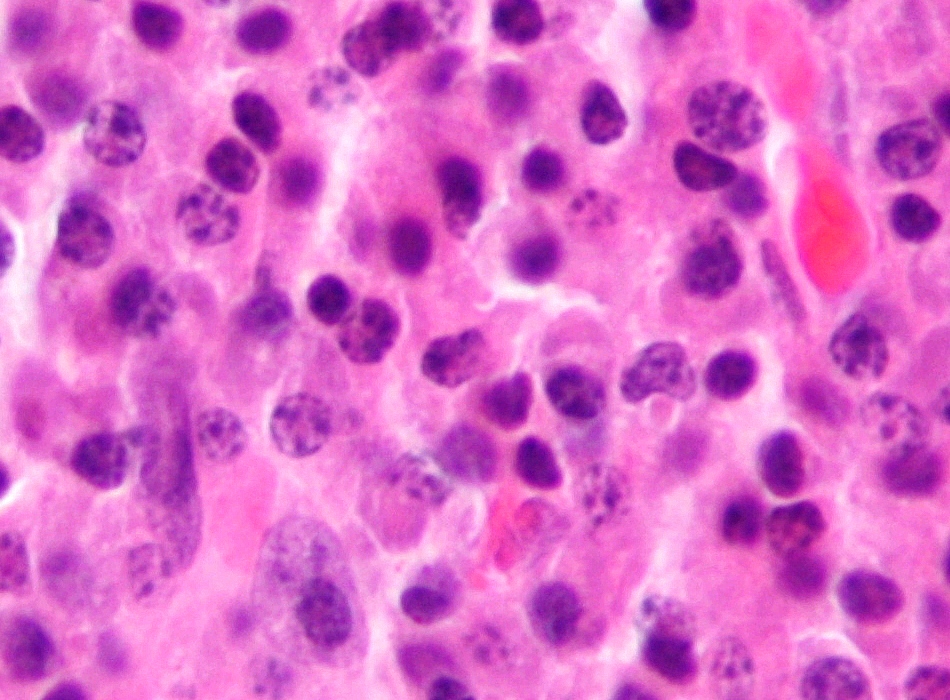
Total, sufferers on this research represented a higher-risk inhabitants, with extra frail people and a higher prevalence of multidrug refractory illness and cytogenetic abnormalities, in keeping with Razzo.
The bispecific antibody diminished illness burden by at the least half in 53% (270) of the 506 evaluable sufferers, with 45% (228) having at the least 90% discount in illness burden (so-called “excellent partial response” within the official response standards).
At a median potential follow-up of 10.1 months, half of sufferers remained freed from development for at the least 5.8 months and an estimated 61% have been alive at one 12 months. Even with the excessive prevalence of sufferers with high-risk options, there seemed to be no improve in hostile occasion frequency in comparison with their prevalence in MajesTEC-1 and different real-world analyses of the bispecific antibody’s use.
Notably, the 56 sufferers who would have been eligible for MajesTEC-1 had comparable general response charges in contrast with the registration trial inhabitants, 61% and 63%, respectively.
With regard to MajesTEC-1-ineligible sufferers, the bispecific antibody additionally benefited many sufferers beforehand handled with BCMA-targeting CAR T cells or the antibody-drug conjugate belantamab mafodotin (Blenrep). Forty % exhibited “excellent partial responses,” together with 43% of the 58 sufferers whose illness had been beforehand handled with the antibody-drug conjugate and 38% of the 104 sufferers whose illness had beforehand been handled with CAR T cells.
Additional analyses revealed that sufferers who underwent prior BCMA-targeting remedy inside 9 months of beginning teclistamab-cqyv exhibited decrease charges of “excellent partial responses” and shorter durations of progression-free survival.
Nonetheless, this therapeutic resistance occurred extra usually within the group lately handled with CAR T cells than in these lately handled with belantamab mafodotin, who responded at a price corresponding to BCMA therapy-naïve sufferers.
“These findings in sufferers with prior BCMA CAR T cell publicity recommend that elevated spacing could enable for the restoration of T-cell health or the reemergence of BCMA-expressing subclones. Alternatively, an extended interval could merely mirror much less aggressive illness biology,” defined Razzo.
In depth pretreatment bone marrow infiltration by myeloma cells (60% fraction or greater) or oblique markers of excessive illness burden corresponding to anemia, thrombocytopenia, or low absolute lymphocyte depend have been additionally considerably related to decrease charges of “excellent partial responses” and shorter durations of progression-free survival.
The research additionally discovered that elevated baseline ferritin was related to inferior outcomes independently of illness burden.
“However, teclistamab-cqyv stays an necessary remedy possibility for sufferers with late-line, relapsed or refractory a number of myeloma, and ought to be thought of even in these with prior BCMA publicity or markers of excessive illness burden and irritation,” stated Razzo.
“Our outcomes spotlight the complicated interaction between real-time medical parameters and baseline illness options in influencing affected person outcomes and recommend that the previous could also be a extra dependable indicator of illness biology than the latter in these sufferers, however there may be nonetheless quite a bit to study,” she added.
To that finish, Razzo and her colleagues are targeted on their ongoing Section II trial investigating limited-duration drug dosing in sufferers with superior a number of myeloma.
Limitations of the research embody the nonstandardized nature of the real-world knowledge in addition to the shortage of a centralized unbiased evaluate or adjudication course of for response and toxicity assessments.
Info relating to the dose depth of teclistamab-cqyv given to sufferers was additionally not out there for evaluation.
Extra info:
Beatrice M. Razzo, et al. Actual-World Expertise with Teclistamab for Relapsed/ Refractory A number of Myeloma from the U.S. Myeloma Immunotherapy Consortium, Blood Most cancers Discovery (2025). DOI: 10.1158/2643-3230.BCD-24-0354
Quotation:
Knowledge present teclistamab can profit many a number of myeloma sufferers who would have been ineligible for pivotal trial (2025, July 9)
retrieved 9 July 2025
from https://medicalxpress.com/information/2025-07-teclistamab-benefit-multiple-myeloma-patients.html
This doc is topic to copyright. Aside from any honest dealing for the aim of personal research or analysis, no
half could also be reproduced with out the written permission. The content material is offered for info functions solely.