Key takeaways:
- Two open-label scientific trials had been carried out to find out efficacy of taletrectinib.
- The advisable day by day dose for taletrectinib is 600 mg orally, with no meals consumption 2 hours earlier than or after remedy.
The FDA has authorized taletrectinib, a ROS1 tyrosine kinase inhibitor, for the remedy of sufferers with regionally superior or metastatic ROS1-positive non-small cell lung most cancers.
The approval of taletrectinib (Ibtrozi, Nuvation Bio Inc.) was primarily based on two open-label scientific trials, TRUST-I and TRUST-II, which measured efficacy of the drug amongst 157 sufferers naive to ROS1 TKI remedy (TRUST-I, n = 103; TRUST-II, n = 54) and 113 sufferers who has acquired such remedy at the very least as soon as (TRUST-I, n = 66; TRUST-II, n = 47).

Information derived from press launch.
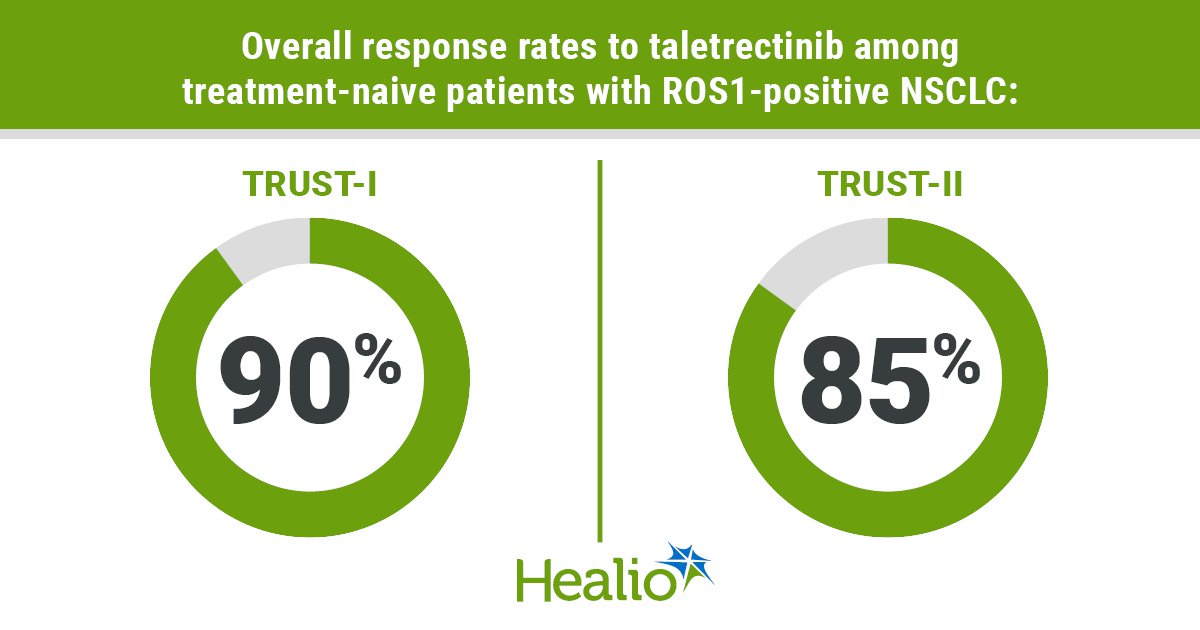
Outcomes confirmed that treatment-naive sufferers had an general response charge of 90% (95% CI, 83%-95%) in TRUST-I and 85% (95% CI, 73%-93%) in TRUST-II. These beforehand handled had an ORR of 52% (95% CI, 39%-64%) in TRUST-I and 62% (95% CI, 46%-75%) in TRUST-II.
Amongst responders, length of response lasted 12 months or longer amongst 72% of treatment-naive sufferers in TRUST-I and 63% in TRUST-II. For the beforehand handled inhabitants, length of response lasted at the very least 6 months amongst 74% of responders from TRUST-I and 83% from TRUST-II.
The FDA-recommended dose of taletrectinib is 600 mg taken orally as soon as per day, with no meals consumption 2 hours earlier than and after the dosage.
The prescribing data for taletrectinib consists of warnings in regards to the dangers for hepatotoxicity, interstitial lung illness/pneumonitis, QTc interval prolongation, hyperuricemia, myalgia with creatine phosphokinase elevation, skeletal fractures and embryo-fetal toxicity.