Need to keep on high of the science and politics driving biotech as we speak? Enroll to get our biotech publication in your inbox.
Good morning. It’s exhausting to be the primary firm in a brand new illness space or drug class, and even perhaps tougher to take care of a lead. We dig into that as we speak.pharma
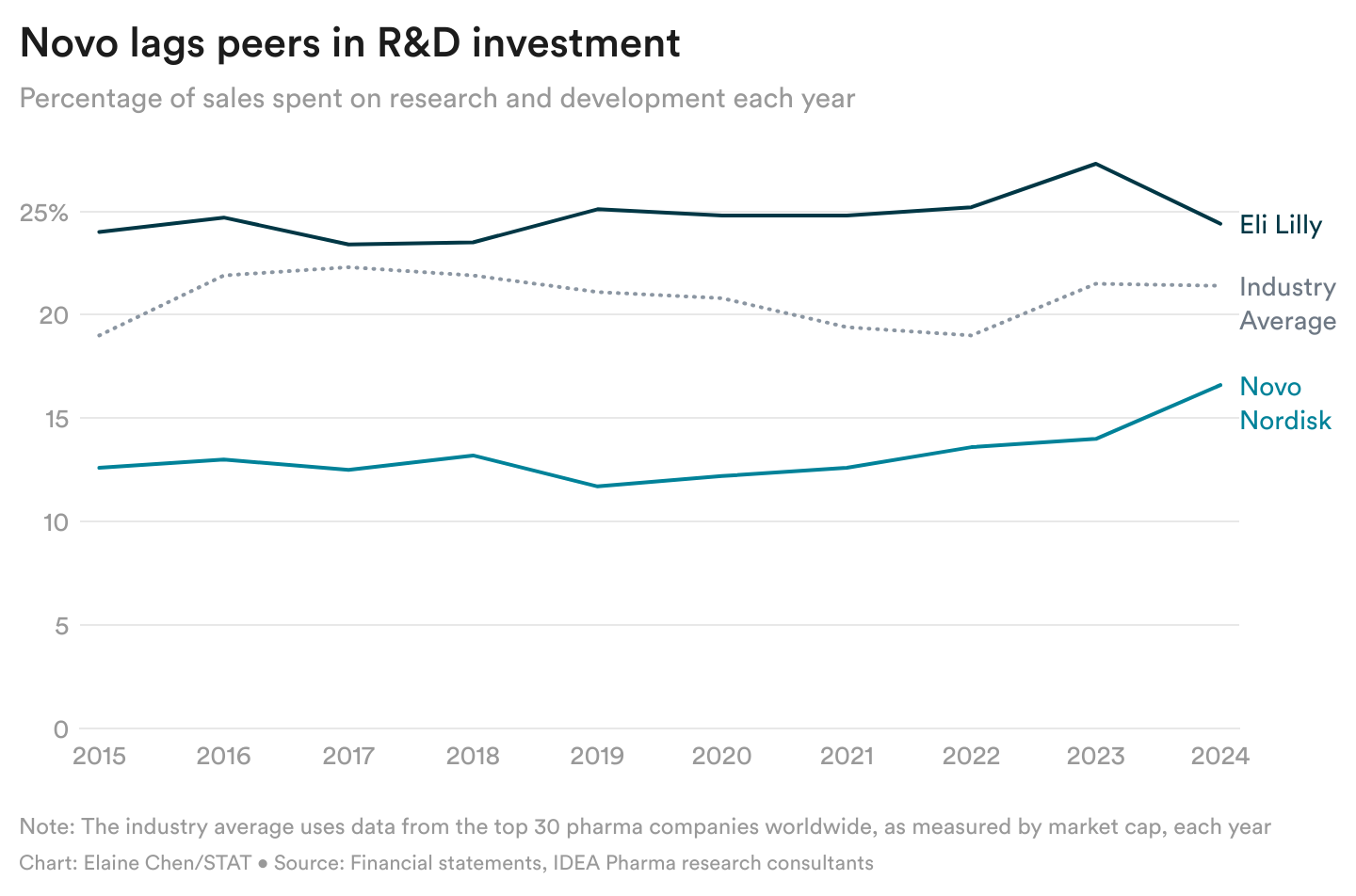
A cautious tradition price Novo its weight problems lead
Danish drugmaker Novo Nordisk was the primary to promote a brand new era of GLP-1 weight problems medication, however it’s now extensively seen as trailing Eli Lilly. How did it get right here? I spoke with former Novo workers to perceive.
Three former R&D workers, talking with me on situation of anonymity, stated Novo took a very cautious method as Lilly solid forward aggressively. For instance, Novo was growing a weekly triple agonist candidate as early because the late 2010s, however it shelved the remedy as Lilly shortly superior its personal triple agonist, referred to as retatrutide.
The previous employees cited varied the reason why they assume Novo was cautious: its conservative tradition and historical past of investing much less in R&D than friends; its perception in Wegovy, which can have blinded it to different drug candidates; and its need to deal with sufferers responsibly, provided that it was the primary to launch a brand new form of weight problems drug.
“Regardless of the C-suite and the manager management speaking all in and prioritizing weight problems and desirous to do one thing totally different, their actions didn’t mirror that messaging by way of placing up inertia into artistic issues” that workers steered, one of many them stated.
RegenxBio reviews new purposeful knowledge on its Duchenne gene remedy
From my colleague Adam Feuerstein: RegenxBio reported an replace this morning from an ongoing scientific trial of RGX-202, its experimental gene remedy for Duchenne muscular dystrophy. 5 boys, ages 6-12, confirmed a median 4.8-point enchancment on the North Star Ambulatory Evaluation, a composite measure of muscle operate, in comparison with a pure historical past management matched for age and baseline muscle operate.
Optimistic outcomes had been additionally reported in different exams designed to measure how briskly it took sufferers to stroll 10 meters, how shortly they may rise off of the ground, and a timed stair-climbing check. 5 boys had been assessed 9 months after receiving RGX-202. RegenxBio additionally reported related enhancements in muscle operate from 4 of the boys who had been adopted for one 12 months. The final purposeful replace from the examine was reported in November 2024.
There have been no extreme unwanted effects, together with liver toxicity, muscle irritation or infections, reported within the examine replace. Sufferers skilled minor nausea, vomiting, and fatigue that resolved.
If profitable, the Regenxbio gene remedy may turn into the second genetic medication for Duchenne to achieve the market, following the approval of Sarepta Therapeutics’ Elevidys in June 2023. The loss of life of an Elevidys affected person in March attributable to liver toxicity has raised security issues concerning the remedy and slowed its uptake.
RegenxBio designed RGX-202 to supply a type of microdystrophin that’s extra like pure dystrophin and probably extra sturdy and efficient. Up to date biomarker knowledge reported this morning confirmed RGX-202 able to producing increased ranges of microdystrophin than Sarepta’s remedy.
The corporate continues to enroll Duchenne sufferers into the examine, with expectations that full, top-line outcomes will likely be obtainable within the first half of subsequent 12 months. If profitable, RegenxBio intends to file for approval in the midst of 2026.
Lilly continues to stay with twin and triple agonists
Talking of weight problems — Eli Lilly is trying to Swedish biotech Camurus to develop long-acting therapies.
Camurus stated earlier this week that beneath a brand new deal, Lilly can use its expertise, referred to as FluidCrystal, to develop and commercialize as much as 4 medication chosen from a pool of candidates that embody twin and triples agonists focusing on a mix of GLP-1, GIP, and glucagon, in addition to amylin receptor agonists.
Underneath the deal, Camurus is eligible to obtain as much as $290 million in upfront and milestone funds, in addition to $580 million in sales-based milestone funds and royalties.
Merck will get a lift in Keytruda patent dispute
A U.S. Patent and Trademark Workplace panel agreed to rethink a patent granted to Halozyme Therapeutics that might have an effect on Merck’s plans to broaden the usage of Keytruda.
Merck has been planning to promote a brand new injectable model of Keytruda. Llast 12 months, it petitioned the patent workplace to rethink seven patents that had been awarded to Halozyme associated to sure enzymes that the biotech developed to allow the administration of medicine by injection.
Patent challenges occur on a regular basis, however the battle over Keytruda is extra carefully watched than most because the stakes are so excessive for Merck. Patent safety for the most cancers remedy, which generated $29.5 billion in income final 12 months, lapses in 2028.
Learn extra from STAT’s Ed Silverman
Vigil’s uncommon mind illness drug fails examine
Vigil Neuroscience stated yesterday that its candidate to deal with a uncommon mind illness failed a Part 2 trial.
The drug, iluzanebart, confirmed no helpful results on biomarker or scientific efficacy endpoints in sufferers with adult-onset leukoencephalopathy with axonal spheroids and pigmented glia, or ALSP.
Vigil introduced final month that it’s being acquired by Sanofi for $470 million, however the deal was centered on the biotech’s experimental Alzheimer’s drug and excluded iluzanebart.
Each iluzanebart and Vigil’s Alzheimer’s candidate goal TREM2, a protein thought to spice up the neuroprotective capability of microglial cells within the mind. Final 12 months, an antibody remedy that additionally focused TREM2, developed by Alector and licensed to AbbVie, failed to sluggish the development of Alzheimer’s in a Part 2 examine.