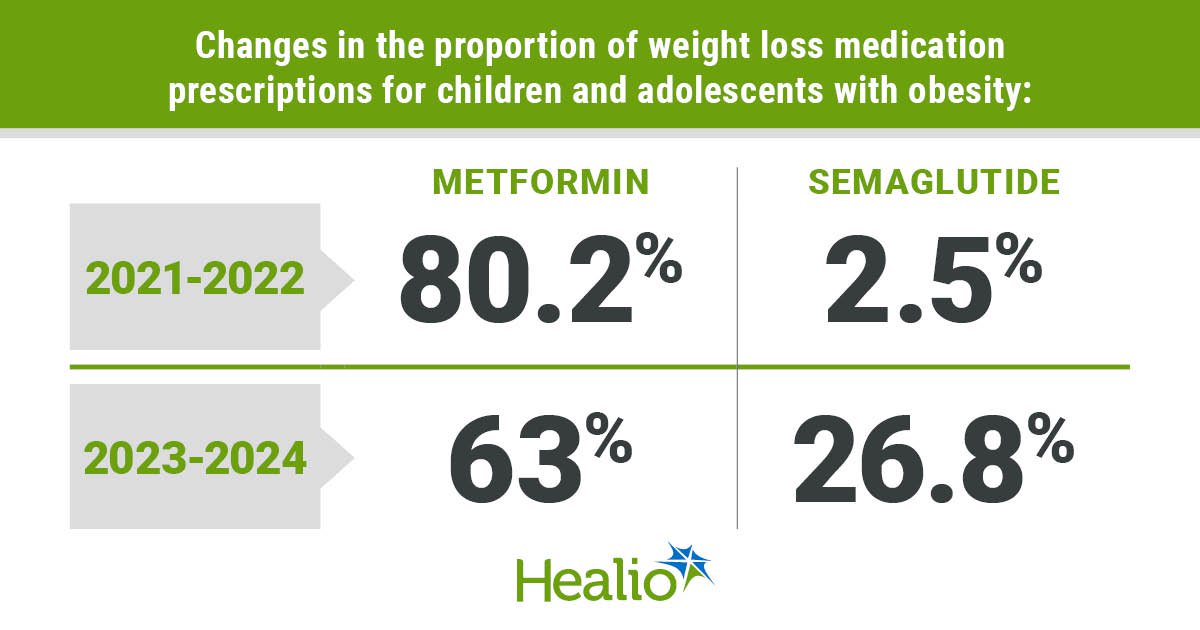
Good morning, everybody, and welcome to a different working week. We hope the weekend respite — which was longer than typical on our aspect of the pond, due to a vacation — was stress-free and invigorating. Now, although, that oh-so-familiar routine of conferences, deadlines, and messages has returned. However what are you able to do? There isn’t a pause button to cease the world from spinning. So this implies one factor: time to dig in to the duties at hand. On that observe, we have now assembled a menu of tidbits that will help you get began. In the meantime, we have now additionally fired up the espresso kettle for an additional cup of stimulation. Our selection at this time is strawberry creme. We hope your day is just smashing, and, as at all times, do communicate if one thing saucy arises. …
Roche is planning to maneuver a brand new antibiotic into late stage medical trials after early research confirmed it had potential to sort out a typical superbug that has grow to be immune to different therapies, The Monetary Occasions says. If profitable, it might be the primary new class of antibiotic able to killing acinetobacter or another “Gram-negative” micro organism to be developed for greater than 50 years. Gram-negative micro organism are notably onerous to deal with as a result of they’ve a second outer membrane, making a formidable barrier for medicine to cross. The final new class of antibiotics authorised to deal with Gram-negative micro organism was in 1968. Roche will launch a Section 3 trial for zosurabalpin on the finish of the 12 months, or early subsequent 12 months. Acinetobacter may cause life-threatening infections together with pneumonia and sepsis, and sufferers who’re immunocompromised due to most cancers or different critical illnesses are notably weak. The trial will recruit about 400 sufferers at greater than 100 websites worldwide, with the purpose of getting the drug authorised towards the tip of the last decade.
Colorado’s prescription drug affordability board members indicated they plan to make use of Medicare’s negotiated value for the autoimmune therapy Enbrel as a key benchmark for setting a restrict on what well being plans within the state pay for the remedy, Bloomberg Regulation writes. Board members stated of their first rule-making listening to on setting an higher fee restrict for Amgen‘s top-selling drug that they might set a cap at or above Medicare’s annual most truthful value of roughly $30,000 per affected person for 2026. The Medicare negotiated value for Enbrel set underneath the Inflation Discount Act drug value program is ready to take impact subsequent 12 months. The pharmaceutical business, nevertheless, has cautioned in opposition to utilizing the Medicare value for setting an higher fee restrict. Amgen misplaced a authorized battle in March, when a U.S. courtroom decide dominated that the Colorado board can proceed with plans to put limits on the costs paid for medicines, which was the primary such determination to help the controversial makes an attempt by some states to regulate their prescription drug spending.


This text is unique to STAT+ subscribers
Unlock this text — plus in-depth evaluation, newsletters, premium occasions, and information alerts.
Have already got an account? Log in