Key takeaways:
- Including inavolisib to palbociclib and fulvestrant considerably prolonged OS for sure PIK3CA-mutated superior breast cancers.
- The routine additionally considerably delayed the time to chemotherapy.
The addition of inavolisib to palbociclib and fulvestrant considerably prolonged OS for sure sufferers with superior breast most cancers, based on outcomes of a randomized part 3 trial scheduled for presentation at ASCO annual assembly.
Adults with beforehand handled endocrine-resistant, PIK3CA-mutated, hormone receptor-positive, HER2-negative illness who obtained inavolisib (Itovebi, Genentech) had a 33% decrease threat for loss of life in contrast with those that didn’t.

Information derived from Turner NC, et al. Summary 1003. Offered at: ASCO Annual Assembly; Might 30-June 3, 2024; Chicago.
“That is the primary time total survival has been considerably improved by a PI3K pathway-targeted drug,” Nicholas C. Turner, MD, PhD, FRCP, professor and director of scientific analysis and growth at The Royal Marsden NHS Basis Belief and Institute of Most cancers Analysis, mentioned throughout a press briefing.
INAVO120 trial
About 70% of all breast most cancers instances within the U.S. contain sufferers with HR-positive, HER2-negative illness, based on an ASCO press launch.
Roughly 40% of that group have a PIK3CA mutation, which prior analysis has proven is related to poor prognosis.
“In these cancers, the estrogen receptor, CDK4/6, and PI3K signaling pathways are key to most cancers development and survival,” Turner mentioned. “Focusing on these three pathways without delay with totally different medicine can improve responses to the remedy and in addition delay resistance. Beforehand, it has not been doable to mix PI3K inhibitors with CDK4/6 inhibitors as a consequence of unintended effects.”
In October 2024, the FDA authorized inavolisib, a PIK3 inhibitor, plus palbociclib (Ibrance, Pfizer) and fulvestrant as remedy for sufferers with endocrine-resistant, PIK3CA-mutated, hormone receptor-positive, HER2-negative superior breast most cancers whose illness recurred on or after ending adjuvant endocrine remedy.
The FDA primarily based its approval on prior findings of INAVO120, which included 325 sufferers (median age, 54 years; vary, 27-79; 98.2% girls; 58.8% white; 38.2% Asian) who had illness recurrence inside 12 months of adjuvant endocrine remedy and had not obtained prior systemic remedy for regionally superior or metastatic illness.
Researchers randomly assigned sufferers 1:1 to 9 mg oral inavolisib or placebo as soon as every day, plus 125 mg oral palbociclib for 21 straight days adopted by 7 days off. Sufferers additionally obtained 500 mg fulvestrant administered intramuscularly on days 1 and 15 of the primary 28-day cycle, adopted by day 1 of each subsequent cycle.
The inavolisib arm had 161 sufferers and the placebo had 164.
Healio beforehand reported part 3 outcomes, which confirmed adults within the inavolisib arm had a considerably longer PFS (median, 15 months vs. 7.3 months; stratified HR = 0.43; 95% CI, 0.32-0.59) and period of response (18.4 months vs. 9.6 months; HR = 0.57; 95% CI, 0.33-0.99).
PFS served as the first endpoint. OS, goal response price, greatest total response, scientific profit price, period of response and patient-reported outcomes served as secondary endpoints.
‘Huge step ahead’
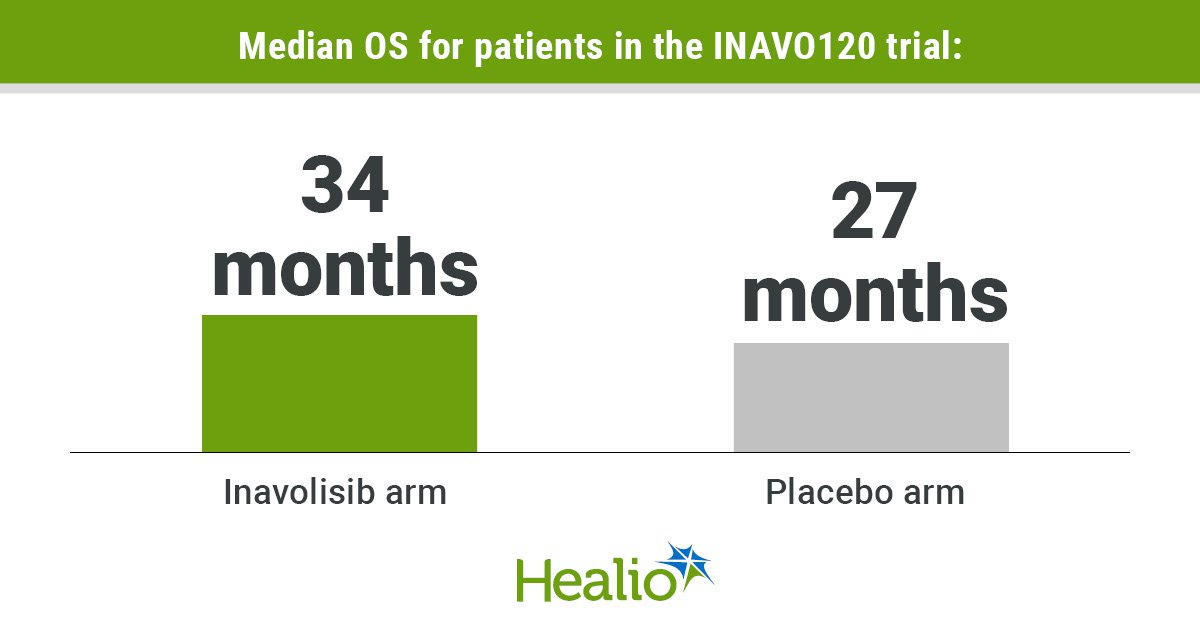
At median follow-up of 34.2 months, sufferers within the inavolisib arm had considerably longer median OS (34 months vs. 27 months; stratified HR = 0.67; 95% CI, 0.48-0.94) in contrast with these within the placebo group.
Those that obtained inavolisib additionally had a better chance of being alive 6 months after remedy (96.8% vs. 90.1%), in addition to at 1 12 months (87% vs. 76.7%), 1.5 years (74.3% vs. 67.2%), 2 years (65.8% vs. 56.3%) and a pair of.5 years (56.5% vs. 46.3%).
Moreover, the investigative arm had a superior goal response price (62.7% vs. 28%) and began chemotherapy later (median, 35.6 months vs. 12.6 months; stratified HR = 0.43; 95% CI, 0.3-0.6).
Researchers additionally discovered members who obtained inavolisib had a “substantial” PFS enchancment from the earlier replace, Turner mentioned. Median PFS improved from 15 months to 17.2 months within the inavolisib arm however didn’t change for the placebo arm (7.3). The inavolisib arm had a 58% longer PFS than the placebo group (stratified HR = 0.42; 95% CI, 0.32-0.55).
Sufferers who obtained inavolisib had greater charges of grade 3 or 4 antagonistic occasions (90.7% vs. 84.7%), critical antagonistic occasions (27.3% vs. 13.5%) and any-grade hyperglycemia (63.4% vs. 13.5%).
In addition they discontinued remedy as a consequence of antagonistic occasions (6.8% vs. 0.6%) or lowered dose (14.9% vs. 3.7%) extra typically.
Turner described the security profile as “manageable,” however cautioned that inavolisib is just not with out toxicities, highlighting hyperglycemia, rash and diarrhea.
“We’ve been making an attempt to develop PI3K inhibitors for a few years, and what we’ve realized about it’s the extra particular and selective your drug can get for the mutant protein, the more practical it’s as a result of it minimizes unintended effects,” he mentioned. “Inavolisib targets PI3K, but it surely selectively degrades the mutant kinase. This probably makes it extra selective for the breast most cancers that’s acquired the PI3K mutation, in order that maximizes your means to hit PI3K within the tumor, comparatively sparing regular cells within the physique and lowering unintended effects.”
Julie R. Gralow, MD, FACP, FASCO, ASCO chief medical officer, mentioned she was “impressed’ how the triplet routine delayed the necessity for chemotherapy.
Within the press launch, Jane L. Meisel, MD, FASCO, ASCO skilled and co-director of breast medical oncology on the Winship Most cancers Institute of Emory College College of Drugs, referred to as the outcomes a “huge step ahead for these sufferers.”
She added, “This research illustrates the significance of genomic testing on the time of analysis of hormone receptor-positive metastatic breast most cancers, in order that sufferers with PIK3CA mutations who qualify for this strategy will be readily recognized.”