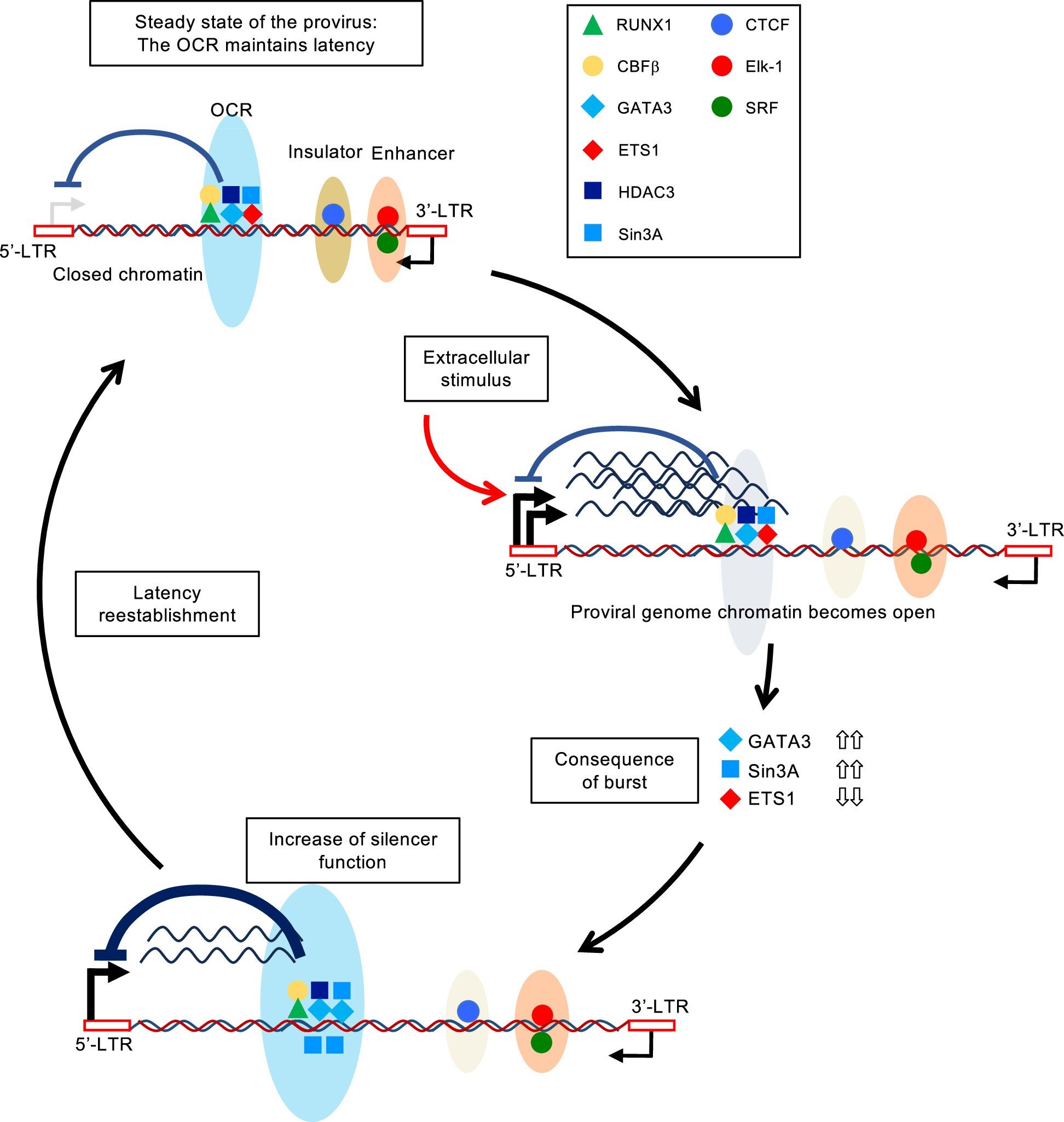
Key takeaways:
- Roflumilast foam 0.3% is a formulation that may deal with psoriasis-affected pores and skin in hair-bearing areas.
- It additionally offers sufferers a nonsteroidal choice that can be utilized long run.
The FDA accepted a supplemental new drug software for roflumilast topical foam 0.3% for the therapy of plaque psoriasis of the scalp and physique for adults and kids aged 12 years and older, in keeping with an trade press launch.
Beforehand, roflumilast cream 0.3% (Zoryve, Arcutis Biotherapeutics) was the one formulation of this drug accepted for psoriasis, making it troublesome to deal with affected areas that had been hair bearing.

“We’re excited to have Zoryve topical foam 0.3% now accepted as a once-daily topical for the therapy of plaque psoriasis of scalp and physique,” Patrick Burnett, MD, PhD, FAAD, chief medical officer at Arcutis Biotherapeutics and an investigator for the part 3 examine supporting the approval, instructed Healio. “This approval gives well being care suppliers a alternative for his or her most well-liked administration and sufferers a alternative on how they wish to have their psoriasis handled.”

Patrick Burnett
The approval was supported by optimistic outcomes from a part 2 trial and the part 3 ARRECTOR trial. Each had been multicenter, randomized, double-blind, vehicle-controlled research that evaluated the security and efficacy of roflumilast foam 0.3% in 736 adults and adolescents aged 12 years and older with gentle to extreme plaque psoriasis of the scalp and physique. In each trials, members had been randomly assigned to roflumilast foam or car foam as soon as each day for 8 weeks. As Healio beforehand reported, outcomes from the part 3 ARRECTOR trial confirmed that members handled with roflumilast noticed increased efficacy outcomes than these handled with the car by week 8.
Within the roflumilast group, 66.4% of members with scalp psoriasis reached IGA success, outlined as an IGA rating of 0 or 1 plus a two-grade enchancment from baseline, in contrast with 27.8% within the car group (P < .001). Equally, 45.5% of these with physique psoriasis within the roflumilast group additionally achieved IGA success vs. 20.1% within the car group (P < .001).
Extra members within the roflumilast group achieved Scalp Itch-Numeric Ranking Scale success, outlined as no less than a four-point enchancment from baseline amongst these with a baseline rating of 4 or larger, at week 2 (25.2% vs. 8%), week 4 (46.2% vs. 16.8%) and week 8 (65.3% vs. 30.3%) than the car group.
“Once you consider treating any pores and skin situation in a hair-bearing space, it may be actually messy if you’re utilizing one thing like a cream, lotion or ointment,” Jennifer Soung, MD, a dermatologist at Southern California Dermatology, a member of the medical board of the Nationwide Psoriasis Basis and an investigator for the part 3 trial, instructed Healio. “Foam, significantly Zoryve foam, is uniquely designed to be moisturizing, light-weight and simply absorbable for the hair.” Remedy-emergent opposed occasions occurred in 26.7% of members handled with roflumilast vs. 16.6% handled with the car. Of the 75 members who skilled treatment-emergent opposed occasions within the roflumilast group, 72 reported the occasions to be gentle or reasonable in severity, whereas two had been severe.
“Typically a foam might be irritating as a result of there’s a little little bit of alcohol,” Soung stated. “However their crew designed this uniquely in order that there aren’t any irritants or alcohol, which means there is no such thing as a stinging or burning when making use of to different areas of the pores and skin.”
Within the part 2 trial, 56.7% of members handled with roflumilast foam for scalp psoriasis reached IGA success vs. 11% of these within the car group by week 8 (P < .0001). Of these with physique psoriasis, 39% additionally reached IGA success in contrast with 7.4% within the car group (P < .0001).
In line with Soung, roflumilast foam simplifies therapy by permitting sufferers to make use of one product for his or her total physique, together with the scalp. Additionally it is gives a steroid-free choice — one thing sufferers usually request, Soung stated.
“For a few years, sufferers and suppliers had been asking if there was something past topical steroids,” Soung stated. “It has been difficult to invent a brand new topical that penetrates the pores and skin sufficient with out triggering systemic absorption … however with Zoryve, we lastly have an alternative choice to a topical steroid that sufferers can use for so long as they need, wherever on the pores and skin.”
For extra data:
Patrick Burnett, MD, PhD, FAAD, might be reached at media@arcutis.com.
Jennifer Soung, MD, might be reached at media@arcutis.com.