Key takeaways:
- Rademikibart is an IL-4R alpha inhibitor that blocks IL-4 and IL-13 signaling.
- 77% of FEV1 enhancements occurred inside 24 hours.
- Sufferers with excessive eosinophil counts skilled better enhancements.
SAN FRANCISCO — Sufferers with average to extreme bronchial asthma noticed will increase in FEV1 inside 24 hours after taking a loading dose of rademikibart, in response to an summary offered on the American Thoracic Society Worldwide Convention.
This speedy response means that the drug could have use in acute settings, Barry Quart, PharmD, chief govt officer, Join Biopharma, advised Healio.

Information have been derived from Collazo R, et al. Poster 1020. Introduced at: American Thoracic Society Worldwide Convention; Might 16-21, 2025; San Francisco.
“All the biologics authorized for bronchial asthma or COPD, no matter what their goal is, are authorized for and used for persistent upkeep therapy for prevention of future exacerbations,” Quart stated. “Actually, the package deal inserts for the entire biologics authorized for bronchial asthma and COPD explicitly say, ‘shouldn’t be used to deal with acute signs or acute exacerbations of bronchial asthma or COPD.’”

Barry Quart
But information for rademikibart point out dramatic enhancements in airway perform the morning after the primary dose, he continued, including that the greater than one million sufferers with bronchial asthma who go to the ED for an acute exacerbation every year have been handled the identical method for greater than 20 years.
“These sufferers get steroids, and so they get bronchodilators, and a few of them get despatched dwelling. A few of them find yourself within the hospital for a number of days as a result of they will’t get stabilized,” Quart stated. “No one’s growing a biologic for this acute therapy setting.”
As a monoclonal antibody and next-generation IL-4R alpha inhibitor, rademikibart (CBP-201, Join Biopharma) blocks IL-4 and IL-13 signaling.
Quart stated that he and his colleagues have at all times thought of rademikibart to be a second-generation dupilumab (Dupixent; Sanofi, Regeneron), which is one other monoclonal antibody that targets IL-4 and IL-13 signaling. “Nevertheless, they bind very otherwise based mostly on a way more secure molecular construction with the IL-4 receptor complicated,” he stated. “Rademikibart, vs. what’s usually believed with biologics, works very quickly after administration.”
The section 2b trial included 322 sufferers with average to extreme bronchial asthma who obtained 150 mg or 300 mg subcutaneous doses of rademikibart or placebo each 2 weeks for twenty-four weeks, adopted by 8 weeks of follow-up.
The 150 mg group included 106 sufferers (imply age, 51.6 years; 66% girls; 77.4% white), the 300 mg group included 108 sufferers (imply age, 52.7 years; 63% girls; 81.5% white) and the placebo group included 108 sufferers (imply age, 54.8 years; 55.6% girls; 73.1% white).
All sufferers started with a 600 mg loading dose of rademikibart or placebo after which carried out prebronchodilator FEV1 measurements at dwelling every day through the research.
“At 1 week, we had extremely statistically vital, very clinically related enhancements in airway perform throughout all sufferers receiving rademikibart,” Quart stated.
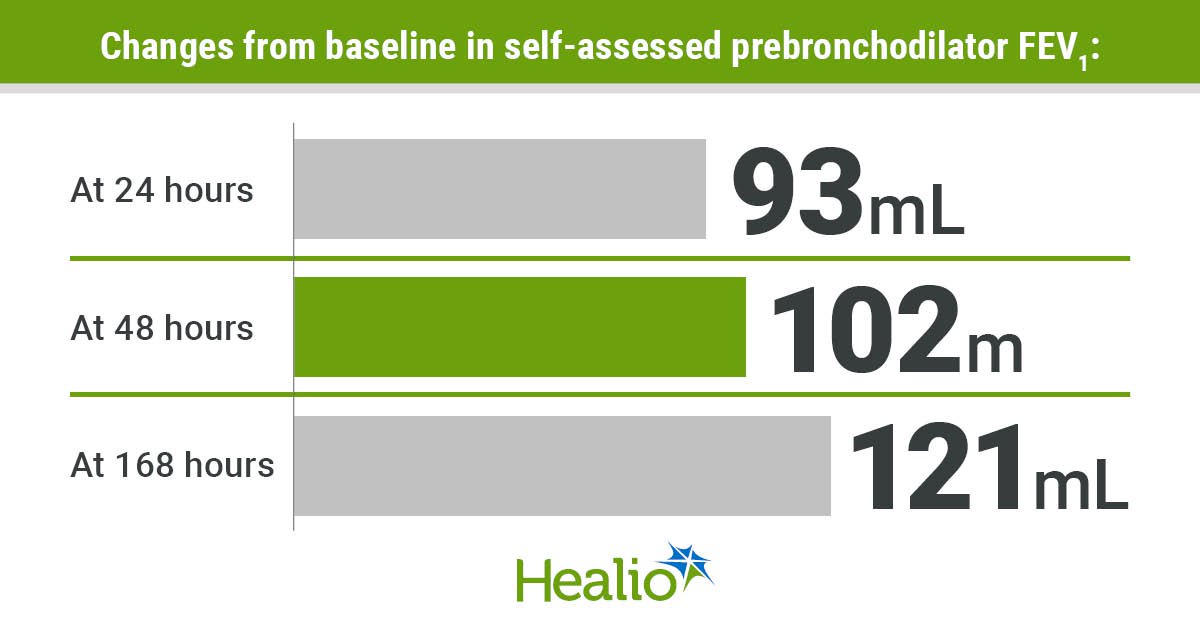
Enhancements in FEV1 for 129 sufferers within the cohort with T2-mediated bronchial asthma, outlined by baseline eosinophil counts of 300 cells/L or larger, included 93 mL after 24 hours, 102 mL after 48 hours, and 121 mL after 168 hours, or 1 week. Particularly, 77% of those enhancements occurred inside 24 hours, and 84% of those enhancements occurred inside 48 hours.
That is the inhabitants of bronchial asthma sufferers at present being enrolled within the researchers ongoing section 2 research, Quart stated.
“It was stunning to us that the product labored as rapidly because it does,” Quart stated.
The house spirometry readings have been constantly decrease and extra variable than these carried out in clinic, stated the researchers, attributing them to a doable lack of in-clinic teaching on correct method by medical personnel.
In clinic, the researchers continued, FEV1 measurements improved by roughly 200 mL by the top of the primary week and persevered by means of week 24 for the therapy teams, together with enhancements of 230 mL for the 150 mg group and 279 for the 300 mg group. The placebo group improved by 89 mL by week 24.
“The house spirometry information are positively extra variable than what we get within the clinic. Clearly, if I didn’t have that rock stable 1-week information within the clinic, it might be extra tenuous to simply accept the house spirometry information,” Quart stated.
“However we already know that we’re seeing a number of hundred milliliter enhancements in airway perform at 7 days, and it was unlikely that it began on day 6 or day 5,” he continued. “The magnitude was so nice on day 1, it was fairly arduous to not consider that it’s actual information.”
Enhancements have been better for sufferers with additional markers of inflammatory-based bronchial asthma with baseline eosinophil counts of 300 cells/µL and better and baseline FeNO ranges of 25 ppb and better (n = 90), together with a 132 mL enchancment at 24 hours, 160 mL enchancment at 48 hours and 184 mL enchancment at 168 hours.
Like the general inhabitants, the researchers added, 72% of this enchancment occurred inside the first 24 hours, with 87% of this enchancment occurring inside the first 48 hours.
Amongst sufferers with T2-mediated bronchial asthma, exacerbation charges fell by 63% (P = .02) for the 150 mg group and by 73% (P = .04) for the 300 mg group as properly.
The researchers additionally characterised the treatment-emergent adversarial occasions as comparable between the teams, with most categorized as grade 1 or 2. None of those occasions have been attributable to eosinophilia.
“Dupixent has a stunning impact of accelerating eosinophils, and the underlying reason for that has by no means actually been described within the literature,” Quart stated. “Actually, we see precisely the other. Relatively than a rise in eosinophils, we see a normal lower.”
By group, 72.6% of the 150 mg group, 70.4% of the 300 mg group and 59.3% of the placebo group skilled a minimum of one treatment-emergent adversarial occasion.
4 sufferers within the 150 mg group, three within the 300 mg group and two within the placebo group discontinued therapy attributable to these occasions, all of which have been resolved, and none of which have been associated to therapy.
There additionally have been two critical treatment-emergent adversarial occasions within the 150 mg group, three within the 300 mg group and three within the placebo group, the researchers continued, however none of them have been associated to rademikibart.
Primarily based on these findings, the researchers concluded that sufferers skilled speedy enhancements in lung perform after receiving rademikibart, with most of those enhancements occurring inside the first 24 hours.
Additional, the researchers stated, these findings point out that rademikibart has potential for early therapy amongst sufferers with bronchial asthma pushed by sort 2 irritation after an acute exacerbation with sustained optimistic outcomes.
Subsequent, the researchers will conduct a pair of trials analyzing the usage of rademikibart within the acute therapy of sufferers experiencing an exacerbation.
“The purpose there’s to enhance airway perform in a short time so these sufferers can get out of the hospital and get dwelling,” Quart stated.
Greater than half of sufferers with exacerbations see their exacerbations worsen or have one other exacerbation inside the subsequent 4 weeks, he continued.
“Hospitals don’t receives a commission if a affected person comes again in that 4-week interval,” Quart stated. “It’s necessary to have the ability to present that we will forestall sufferers from returning to the ED, or the hospital, to assist construct the story as to why the hospital ought to pay the cash to make use of this biologic.”
If authorized, rademikibart could be the primary biologic used within the hospital to deal with sufferers with bronchial asthma, he stated. As soon as these exacerbations are underneath management, he added, the researchers hope these sufferers would proceed to make use of rademikibart for upkeep to stop future exacerbations.
“Our market analysis additionally indicated that if the drug labored properly within the acute setting, a big majority of clinicians stated they’d like to maintain the affected person on it chronically,” Quart stated. “That’s the Holy Grail.”
these information, Quart stated, it would work with the FDA to construct a section 3 plan.
“Get the affected person in after they’re having an acute exacerbation, deal with them acutely, after which, after a month, provoke persistent therapy and comply with them for the same old 52 weeks,” he stated.
For extra data:
Barry Quart, PharmD, will be reached at allergy@healio.com.