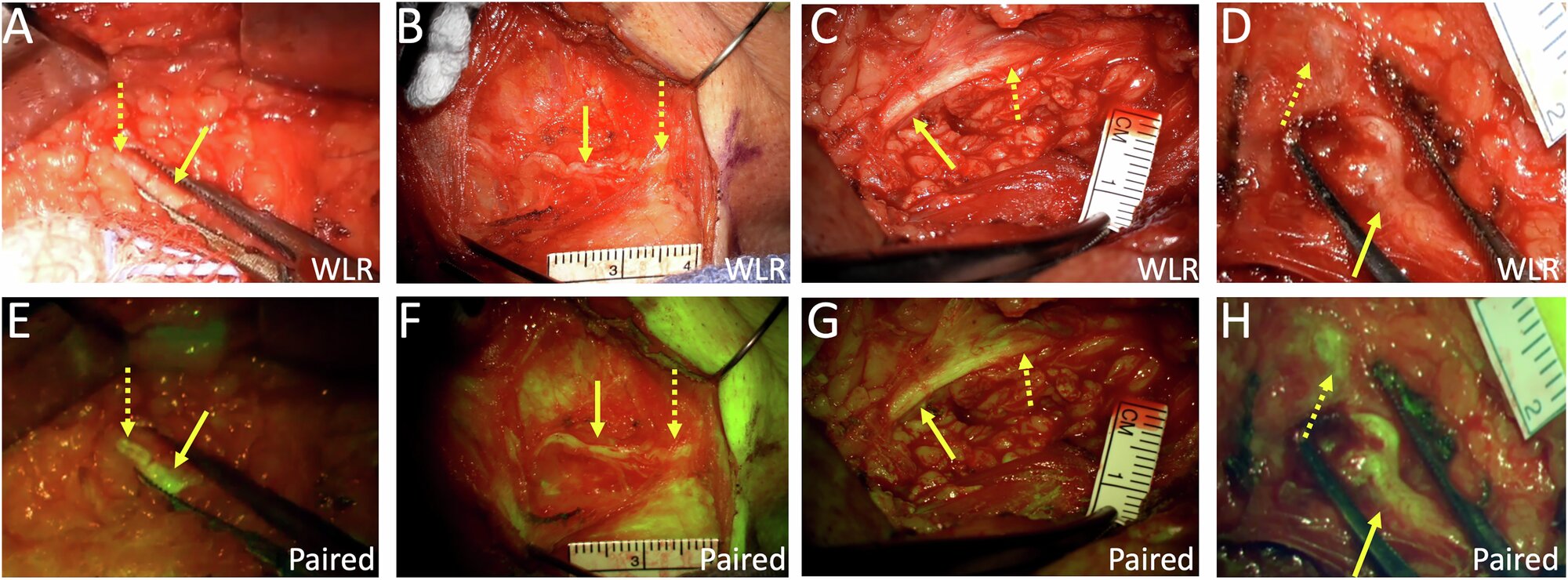
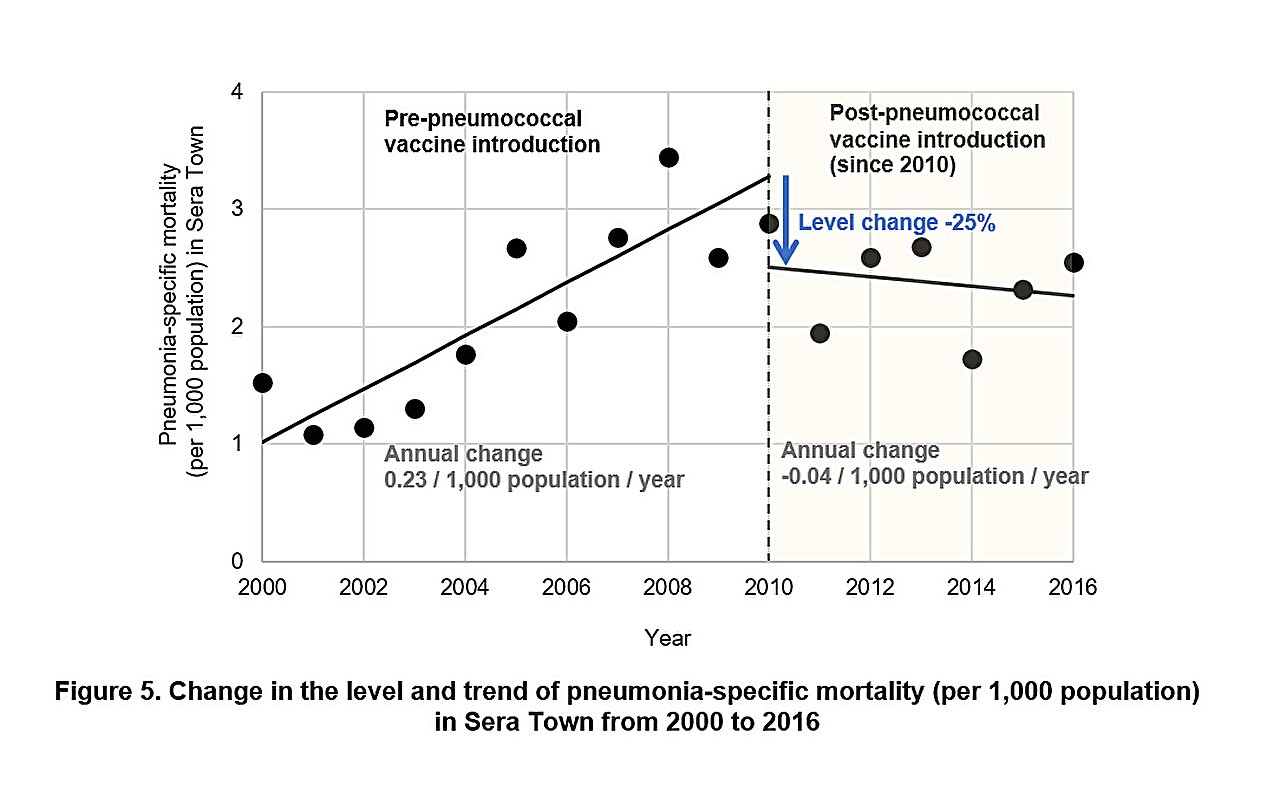
Good morning, everybody, and welcome to a different working week. We hope the weekend respite was stress-free and invigorating, as a result of that oh-so-familiar routine of conferences, deadlines, and messages has returned. However what are you able to do? There isn’t any pause button to cease the world from spinning. So this implies one factor: time to dig in to the duties at hand. On that be aware, we now have assembled a menu of tidbits that will help you get began. In the meantime, we now have additionally fired up the espresso kettle for an additional cup of stimulation. Our selection at present is banana break up. We hope that your day is just smashing and that you just conquer the world. And as all the time, do communicate if one thing juicy and insightful arises. …
The U.S. Meals and Drug Administration has lastly accredited Novavax’s Covid-19 vaccine, however in doing so has positioned restrictions on it that its two opponents within the U.S. market don’t face, STAT tells us. The long-awaited license limits use of the vaccine to individuals 65 and older and folks aged 12 to 64 who’ve a minimum of one medical situation that places them at greater threat of extreme sickness in the event that they contract Covid. The vaccine has been accessible underneath an emergency use authorization. The FDA missed an April 1 deadline to rule on Novavax’s utility and it was reported that political appointees in FDA Commissioner Marty Makary’s workplace overrode profession employees, who advisable issuing the license. Makary has raised considerations about the truth that the scientific trials supporting Novavax’s utility had been carried out a number of years earlier — which isn’t irregular for a vaccine utility — concentrating on a model of the SARS-CoV-2 virus that circulated then.
Regeneron Pharmaceutical reached an settlement to purchase the patron genetics agency 23andMe out of chapter for $256 million, though the deal is topic to chapter courtroom and regulatory approvals, STAT writes. The biotech mentioned it will proceed 23andMe’s client enterprise and use the info the corporate has collected for drug improvement. It mentioned it’s ready to element its meant use of buyer knowledge, together with privateness protections and safety controls, for the assessment of a court-appointed, unbiased buyer privateness ombudsman “and different events.” The deal would seem to bring to a standstill a dramatic sequence of occasions for 23andMe, which grew to become a broadly recognized client model however suffered as a enterprise. It may additionally ease considerations concerning the privateness of 23andMe’s buyer knowledge. With 23andMe coming into chapter in March, stoking uncertainty about its future, specialists have suggested prospects to obtain after which delete their knowledge.


This text is unique to STAT+ subscribers
Unlock this text — plus in-depth evaluation, newsletters, premium occasions, and information alerts.
Have already got an account? Log in