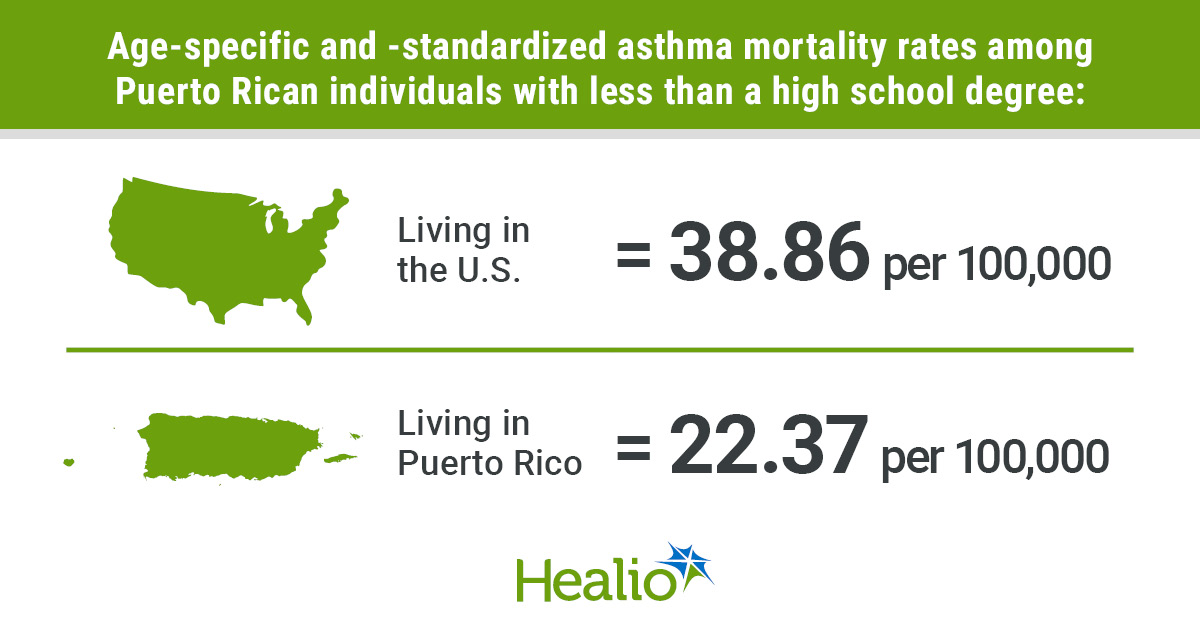
Key takeaways:
- An toddler with a uncommon urea cycle dysfunction turned the primary affected person to obtain a personalised gene-editing remedy.
- His care workforce developed the one-of-a-kind medicine in simply 6 months.
By the second day of his life, Kyle and Nicole Muldoon knew their new child son was very sick.
Throughout a routine examination within the NICU, his physician observed his arm was shaking down slowly after being lifted, reasonably than falling like a typical child. Then, his labs confirmed extraordinarily excessive ranges of ammonia in his blood, indicating that he had a urea cycle dysfunction.

Kyle and Nicole mentioned the next weeks had been a “crash course” in urea cycle deficiencies and their son’s particular situation: carbamoyl phosphate synthetase 1 (CPS1) deficiency.
The toddler’s physician defined that CPS1 deficiency is “an extremely extreme, extremely uncommon illness” through which the liver lacks an enzyme wanted to transform ammonia to urea and excrete it by urination.
“The ammonia buildup may be life-threatening shortly after beginning,” Rebecca C. Ahrens-Nicklas, MD, PhD, a physician-scientist and director of the Gene Remedy for Inherited Metabolic Issues Frontier Program at The Youngsters’s Hospital of Philadelphia, advised reporters throughout a press convention this week. “[He] was fortunate {that a} considerate physician recognized that he had indicators of an elevated ammonia degree, together with poor feeding and sleepiness.”
Ahrens-Nicklas mentioned the Muldoons’ son instantly began dialysis remedies to take away the ammonia from his physique, and the unique plan was to attend till he grew massive sufficient for a liver transplant, which is the same old remedy for his situation.
Later, he transitioned from dialysis to a long-term remedy together with glycerol phenylbutyrate, a nitrogen-scavenger drug, and citrulline supplementation. He consumed a low-protein weight-reduction plan.
Ahrens-Nicklas mentioned CPS1 deficiency is essentially the most extreme type of urea cycle dysfunction, and greater than 50% of infants with the defect will die through the neonatal interval.
“I knew he was going to have a really difficult path, even with glorious care,” she mentioned.
So, she pitched the concept for a gene-editing remedy that had by no means been accomplished earlier than.
“Nicole may be very analytical … and I’m a intestine man,” Kyle Muldoon mentioned through the press convention. “Once we had met with Dr. Ahrens-Nicklas, I had a really profound feeling about this gene enhancing, which was such a overseas idea.”
“To Dr. Ahrens’ credit score, she was so humble in the way in which she was telling us about this extraordinary factor that was primarily going to presumably save and improve our baby’s life,” he mentioned. “We prayed, we talked to folks, we gathered info, and we finally determined that this was the way in which we had been going to go.”
Which is how their son turned the world’s first recipient of a personalised gene-editing remedy in what has been hailed as a medical milestone.
One-of-a-kind remedy
Ahrens-Nicklas teamed up with Kiran Musunuru, MD, PhD, a heart specialist and Barry J. Gertz Professor for Translational Analysis on the College of Pennsylvania Perelman Faculty of Drugs, to research gene-editing therapies 4 years in the past. Musunuru mentioned he has been working for greater than a decade on gene-editing remedy for prime ldl cholesterol.
“We are able to do that by altering the spelling of a ldl cholesterol gene, switching one letter to a different letter so it’s turned off,” he defined through the press convention. “We now have developed a remedy that may be given to a affected person by an IV infusion. It goes straight to the liver, and it turns off the ldl cholesterol gene within the liver, and this remedy is exhibiting nice success in scientific trials.”
Ahrens-Nicklas initially started collaborating with Musunuru in 2021 to develop remedies for phenylketonuria (PKU). Just like the ldl cholesterol remedies his lab was engaged on already, the PKU remedies concerned correcting misspellings — or mutations — in genes to make the liver create enzymes that it was not beforehand creating.
“A little bit greater than 2 years in the past, [Ahrens-Nicklas] actually argued — she didn’t should do a lot to persuade me — that we would have liked to pivot to engaged on these most extreme, devastating ailments: urea cycle issues,” Musunuru mentioned. “She realized that these therapies would possibly be capable of assist the sickest of her sufferers who had horrible ailments so uncommon that every affected person could be the one one on the earth with a selected misspelling, a particular flaw in an enzyme.”
Within the case of the Muldoons’ son, the physicians didn’t have 1 to 2 years to develop a medicine like they’d in earlier trials. Musunuru mentioned they gathered a workforce of educational and business professionals to fabricate a customized medicine inside simply 6 months.
Throughout that 6-month span, the researchers developed a custom-made CRISPR/Cas9 gene-editing medicine that might be delivered utilizing messenger RNA, examined it in animal and cell fashions and obtained approval from the FDA for an investigational new drug utility. CRISPR-based gene enhancing permits scientists to focus on and make fascinating edits to the genome.
“We met early on with the FDA, they usually had been extremely supportive,” Musunuru mentioned. “They acknowledged it was an uncommon circumstance [and] advised us to do pretty much as good a job as we presumably may within the restricted time we had, and they might take it from there.
“They honored that dedication after we formally submitted our utility [and] authorized it in simply 1 week,” he mentioned.
Exceeding expectations
Between age 6 and seven months, Ahrens-Nicklas mentioned the Muldoons’ son obtained a small dose of his customized IV remedy.
“Infusion day was terrifying and thrilling on the identical time,” she mentioned. “It was an incredible second after we had been capable of really maintain in our fingers the remedy — a syringe of the medicine that we had labored so onerous to create.”
Though his mother and father and care workforce had been nervous and excited, Ahrens-Nicklas mentioned the Muldoons’ son was unfazed and slept by the 2-hour infusion.
As a result of the remedy had not been administered to an toddler earlier than, Ahrens-Nicklas mentioned they began with a low dose (0.1 mg/kg) to make sure his security. After the primary dose, he was capable of devour the advisable every day allowance of protein for a child of his measurement. In response to a associated examine printed in The New England Journal of Drugs, his care workforce was additionally capable of scale back his dosage of glycerol phenylbutyrate from 10.1 to eight.1 mL/sq. meter of physique floor space per day after the primary dose, however his glutamine ranges started to rise a number of weeks later, so he was put again onto his unique dose.
Within the examine, Ahrens-Nicklas and colleagues wrote that he obtained a second, bigger dose (0.3 mg/kg) 3 weeks after the primary. After the second dose, his care workforce diminished his glycerol phenylbutyrate dose by half (10.1 to five ml per sq. meter per day).
“We only in the near past gave him his third and ultimate deliberate dose, so we’re nonetheless studying if it had any extra profit,” Ahrens-Nicklas mentioned. “However on daily basis, he’s exhibiting us indicators that he’s rising and thriving.”
Though she mentioned she would by no means use the phrase “remedy,” Ahrens-Nicklas mentioned the toddler’s progress is reassuring. He’s consuming extra protein, just lately had a development spurt, and is taking much less medicine than when he began. She couldn’t say whether or not he’ll ever not want medicine, however “he confirmed us that he’s safer than he was earlier than.”
Nicole Muldoon mentioned her son exceeds her expectations on daily basis. He’s making an attempt new meals, sitting up in his crib by himself and reaching different vital milestones.
“It blows us away much more as a result of we all know what was stacked up in opposition to him within the very starting and the way dangerous of a prognosis it was to start with for him,” she mentioned.
‘The way forward for medication’
Though it’s nonetheless too early to see the long-term results of this bespoke remedy, Peter Marks, MD, PhD, who just lately retired as director of the FDA’s Middle for Biologics and Analysis and Analysis, mentioned the case demonstrated a doable manner ahead in growing personalised medication.
“There may very well be a whole lot to 1000’s of ailments that may very well be handled by an strategy much like [this one],” he wrote. “That’s, the mix of speedy prognosis by genome sequencing and expedited individualized product growth, adopted by administration of the remedy and cautious monitoring of security and efficacy outcomes, may remarkably enhance the lives of individuals affected by these uncommon issues.”
Marks mentioned it might be doable for corporations to develop a remedy for a uncommon illness after which get regulatory approval for the general strategy, which might streamline growth of disease-specific medication and scale back prices.
“I don’t suppose I’m exaggerating once I say that is the way forward for medication,” Musunuru mentioned. “This is step one towards using gene enhancing therapies to deal with all kinds of uncommon genetic issues for which there are at the moment no definitive remedies and for which there are literally only a few remedies at the moment in growth in any respect.”
References:
For extra info:
Rebecca C. Ahrens-Nicklas, MD, PhD, may be reached at ahrensnicklasr@chop.edu.
Kiran Musunuru, MD, PhD, may be reached at kiranmusunuru@gmail.com.