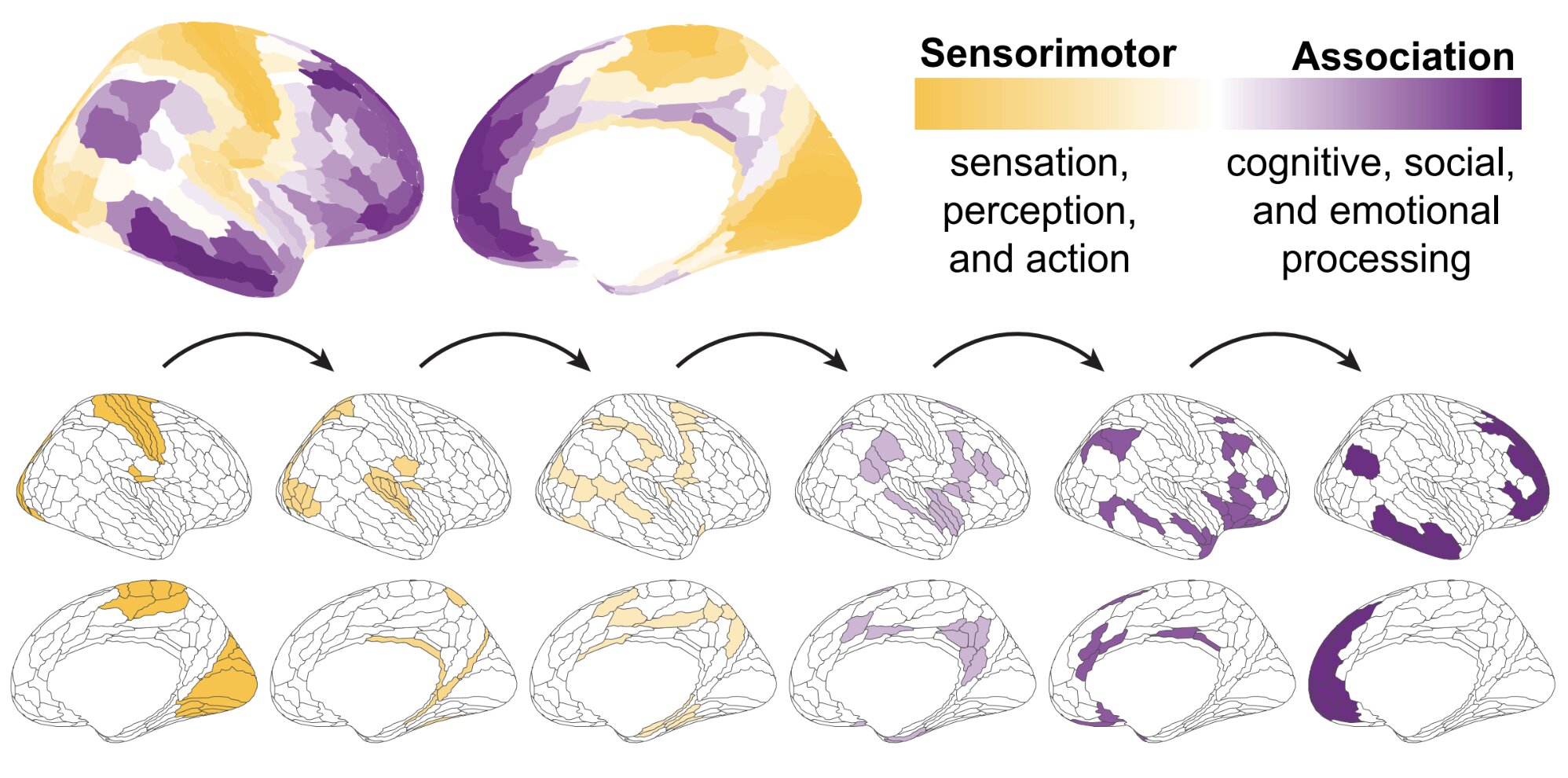
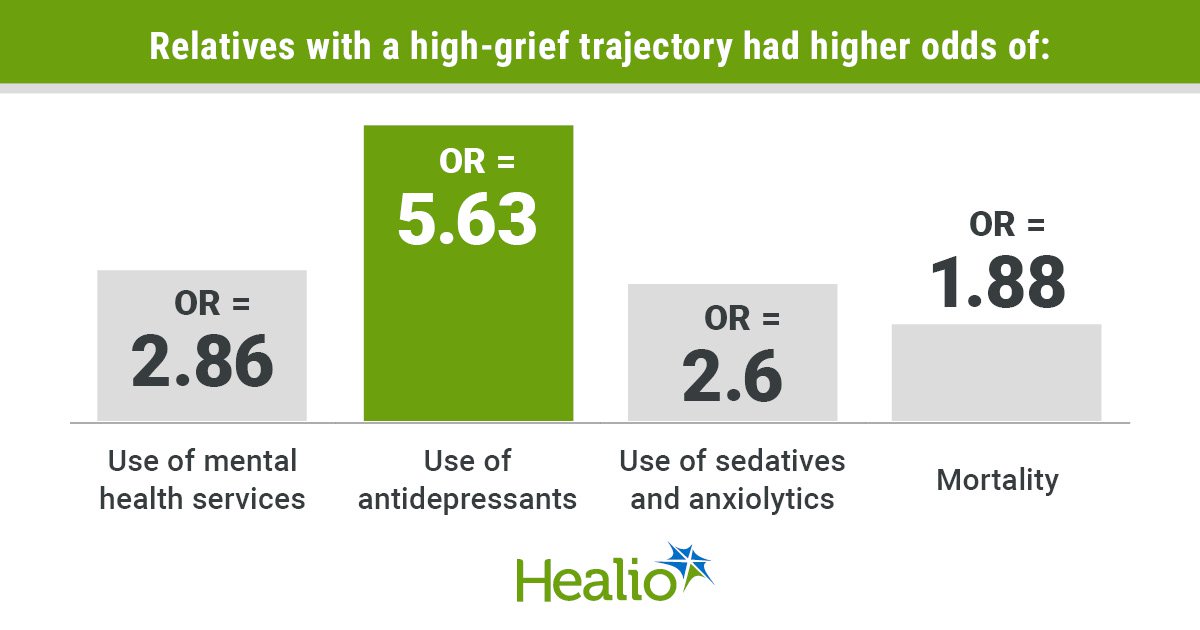
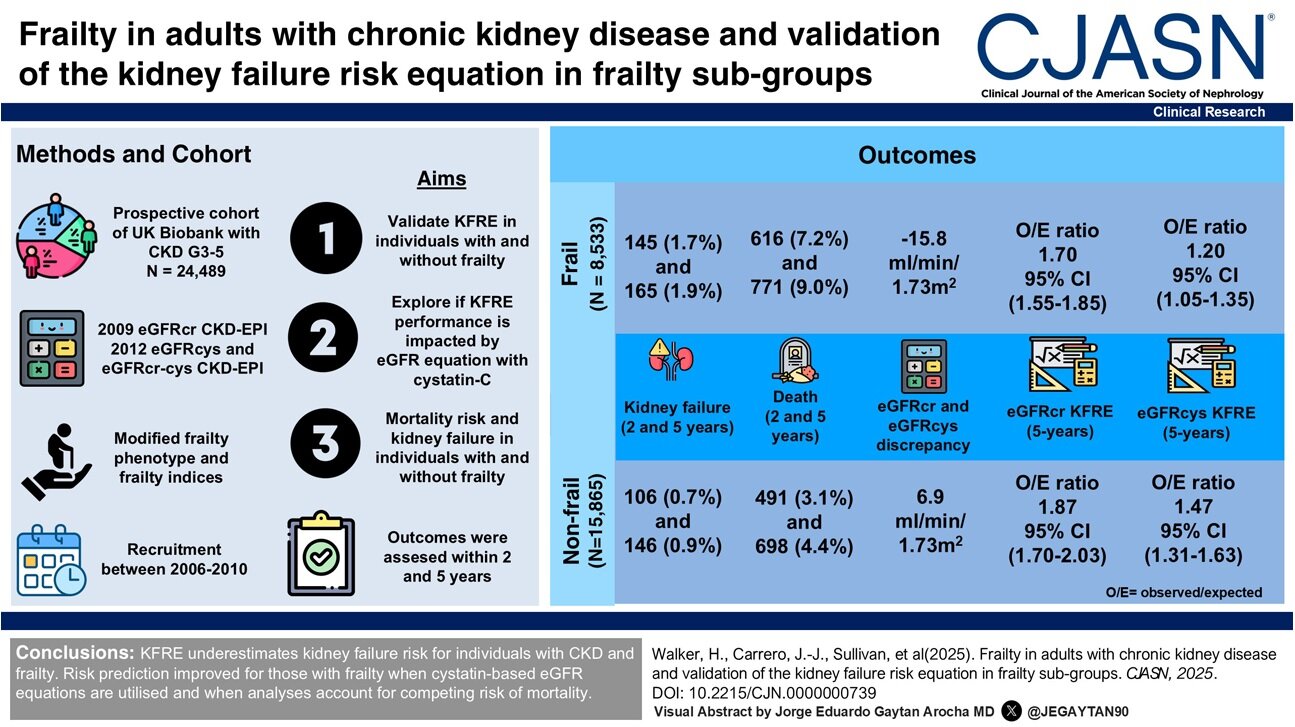
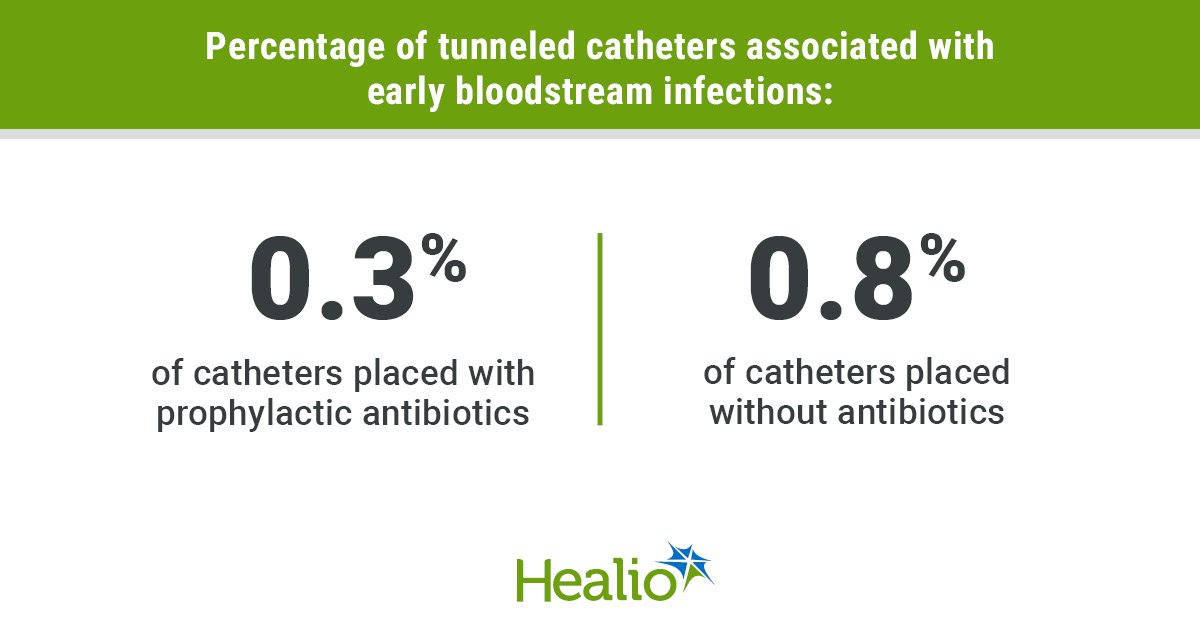
New analysis from Bayes Enterprise College, in collaboration with biopharmaceuticals firm Merck KGaA, suggests member states from the European Union (EU) should work extra carefully collectively, present higher incentives for the event of latest medicines and approve entry to medicines faster than different worldwide regulators, whether it is to draw larger funding from pharmaceutical firms.
The pharmaceutical business is in a extremely aggressive market race for innovation, approval and rollout. Nationwide and regional regulatory frameworks are very important for fostering efficient innovation, creating ecosystems to draw giant pharmaceutical firms to develop organic and orphan medicine regionally.
Regulatory ecosystems, together with these from the US (FDA), European Union (EMA), UK (MHRA), China (NMPA) and Japan (PMDA) are all in competitors to approve progressive medicines, however should additionally work collectively to attain world goals—significantly in crises as seen throughout COVID.
Present tendencies point out that these pharmaceutical firms favor the US and different ecosystems over Europe for first submissions to develop medicine, attributable to sooner common approval instances, regulatory assist and stronger incentives.
The qualitative analysis was led by Stefan Haefliger, Professor of Strategic Administration and Innovation at Bayes, and Pedro Franco, Bayes alumnus (Govt MBA, 2023) and Head of Europe International Regulatory and Scientific Coverage at Merck KGaA. It aimed to discover the reason why main pharmaceutical firms favored different markets, and what the European Union may do to achieve aggressive benefits over these to reestablish its world status. The research concerned interviews with 47 senior practitioners within the prescribed drugs business, from throughout 19 nationalities.
“Competitors of regulatory ecosystems in approving medicines: Coverage implications within the case of Europe” by Pedro Franco and Professor Stefan Haefliger is printed in Drug Discovery Right this moment.
Probably the most generally recognized causes given by consultants for firms selecting the US over the EU included:
- Decrease costs within the EU, which limit revenues and margins for pharmaceutical firms.
- The EU’s smaller market dimension in comparison with the US.
- Issues in post-approval entry to medicines attributable to differing reimbursement techniques throughout EU international locations.
- Rising prices within the EU, together with taxes and analysis & improvement, are disincentivizing manufacturing.
- Inadequate entry to capital, assets and experience.
- Fewer medical trials in European international locations attributable to regulatory restrictions.
The research additionally revealed measures that might assist the European regulatory ecosystem set up aggressive benefits by means of leveraging strengths. These included introduction of regulatory sandboxes, joint scientific recommendation for drug-devices, the introduction of digital product data, and simplification of the present regulatory system and limitless advertising authorization—which avoids pharmaceutical firms to resume licenses each 5 years.
Professor Haefliger mentioned, “It’s well known that the European drug dispersion has fallen behind that of the US and others. Certainly, the UK’s improvement and fast deployment of the Oxford Astra-Zeneca vaccination throughout COVID was celebrated by Eurosceptics as a ‘Brexit dividend’—following the UK regulatory framework’s aggressive technique after departure from the buying and selling bloc.
“Prescribed drugs are additionally a scorching speaking level proper now, with President Trump’s current tariff bulletins inflicting a stir in Eire and throughout the EU—amid fears that business giants similar to Johnson & Johnson could look to relocate as a substitute of paying pricey sums to export their merchandise to the US market.
“Our research is supported by findings of the 2024 Draghi Report, that laid naked the EU’s stagnation in pharmaceutical development and urged reform in regulatory processes, capital entry, and technological adoption.
“Whereas many people in our research cite the dearth of synergy between EU members as a purpose for his or her reluctance to develop medicine in Europe, there stays a extremely various pool of experience—and positively the potential to turn into a extra enticing proposition.
“With out important adjustments, nonetheless, the EU dangers falling additional behind its opponents in attracting pharmaceutical innovation, funding, and main analysis expertise.”
Extra data:
Pedro Franco et al, Competitors of regulatory ecosystems in approving medicines: coverage implications within the case of Europe, Drug Discovery Right this moment (2025). DOI: 10.1016/j.drudis.2025.104295
Quotation:
EU pharmaceutical regulation reforms wanted to drive innovation and funding, consultants counsel (2025, Could 2)
retrieved 4 Could 2025
from https://medicalxpress.com/information/2025-05-eu-pharmaceutical-reforms-investment-experts.html
This doc is topic to copyright. Other than any truthful dealing for the aim of personal research or analysis, no
half could also be reproduced with out the written permission. The content material is offered for data functions solely.