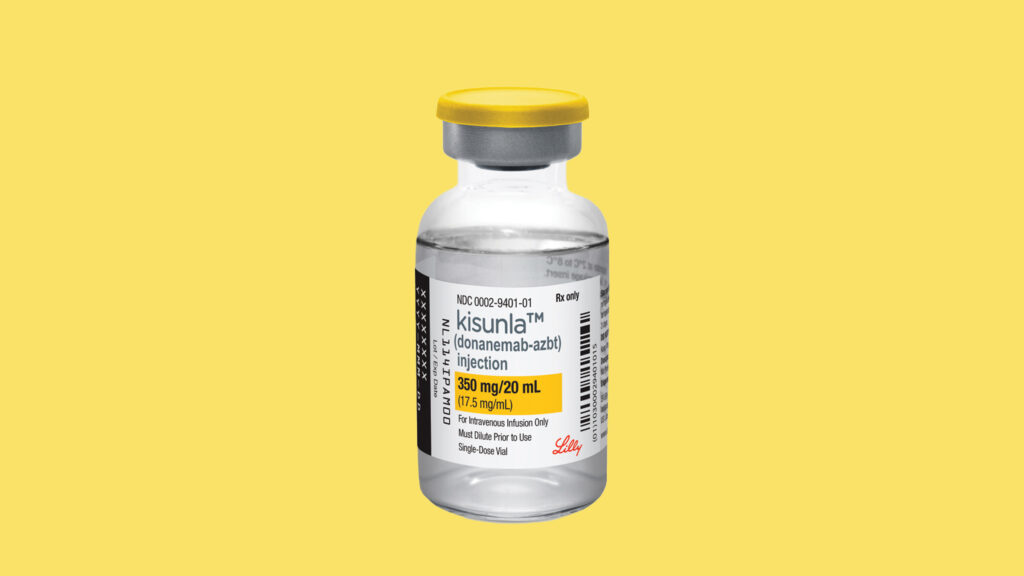
European regulators on Friday stated that Eli Lilly’s Alzheimer’s therapy Kisunla ought to be accredited for a choose group of sufferers, altering course from an preliminary advice to reject the drug.
A European Medicines Company committee dominated in March that the dangers of the remedy — specifically a kind of mind swelling and bleeding often known as ARIA — outweighed the restricted advantages of the therapy, calling for it to be denied.
However following an attraction from Lilly, the committee issued a restricted advice Friday, saying Kisunla ought to be licensed for sufferers whose genetic profile leaves them at decrease danger for ARIA.


This text is unique to STAT+ subscribers
Unlock this text — plus every day protection and evaluation of the pharma trade — by subscribing to STAT+.
Have already got an account? Log in