Key takeaways:
- Injections included six for APG777, 11 for lebrikizumab-lbkz and 10 for dupilumab.
- APG777’s EASI rating discount surpassed different biologics at 16 weeks.
- The protection profile was in keeping with different biologics.
Sufferers with reasonable to extreme atopic dermatitis skilled a 71% lower in Eczema Space and Severity Index scores from baseline by 16 weeks with APG777, an anti-IL-13 antibody from Apogee Therapeutics, in keeping with the corporate.
The biologic achieved this lower and extra constructive outcomes with fewer injections than different biologic therapies as effectively, Michael Henderson, MD, CEO of Apogee, advised Healio.

Information have been derived from press launch.
“IL-13 is the inflammatory cytokine that drives atopic dermatitis and a broad vary of kind 2 inflammatory issues,” Henderson stated.

Michael Henderson
Dupilumab (Dupixent; Regeneron, Sanofi) and lebrikizumab-lbks (Ebglyss, Lilly) additionally goal IL-13 to stop flares and enhance lesions, he continued.
“We’re a really comparable binder to IL-13,” Henderson stated, “simply with a much-improved antibody.”
Different biologics start with dosing each week or each different week earlier than extending to each 4 weeks after which each 8 and 12 weeks, he stated, noting that risankizumab-rzaa (Skyrizi, AbbVie) is a market chief with its quarterly dose schedule.
“In AD, the market chief can solely attain each 2 week dosing, in comparison with different indications like Skyrizi, the place quarterly choices at the moment are obtainable,” Henderson stated.
Apogee has optimized APG777with half-life extension expertise for prime titers that result in an extended half-life within the physique, Henderson stated.
“That helps us to get to about three to 5 occasions that half-life within the physique for our antibody vs. these first-generation antibodies,” he stated. “We will do an injection each 3 to six months, we hope, what takes others each 2 to 4 weeks to do.”
Topline outcomes
Half A of the randomized, placebo-controlled section 2 APEX trial included 123 adults who obtained 720 mg of APG777 at weeks 0 and a pair of earlier than receiving 360 mg at weeks 4 and 12 (n = 82; imply age, 38.7 years; 50% girls; 65.9% white) or a placebo (n = 41; imply age, 36 years; 46.3% girls; 73.2% white). There have been no extra doses of APG777 by 16 weeks.
With six injections over 4 dosing days, the corporate stated APG777 is much less demanding than 11 injections for lebrikizumab-lbkz and 10 injections for dupilumab, each over 9 dosing days by 16 weeks.
Least squares imply (LSM) % modifications in EASI from baseline at week 2 included 35.4% for the therapy group and 20.8% for the placebo group (P < .05). At week 16, LSM % modifications included 71% for the therapy group and 33.8% for the placebo group (P < .001).
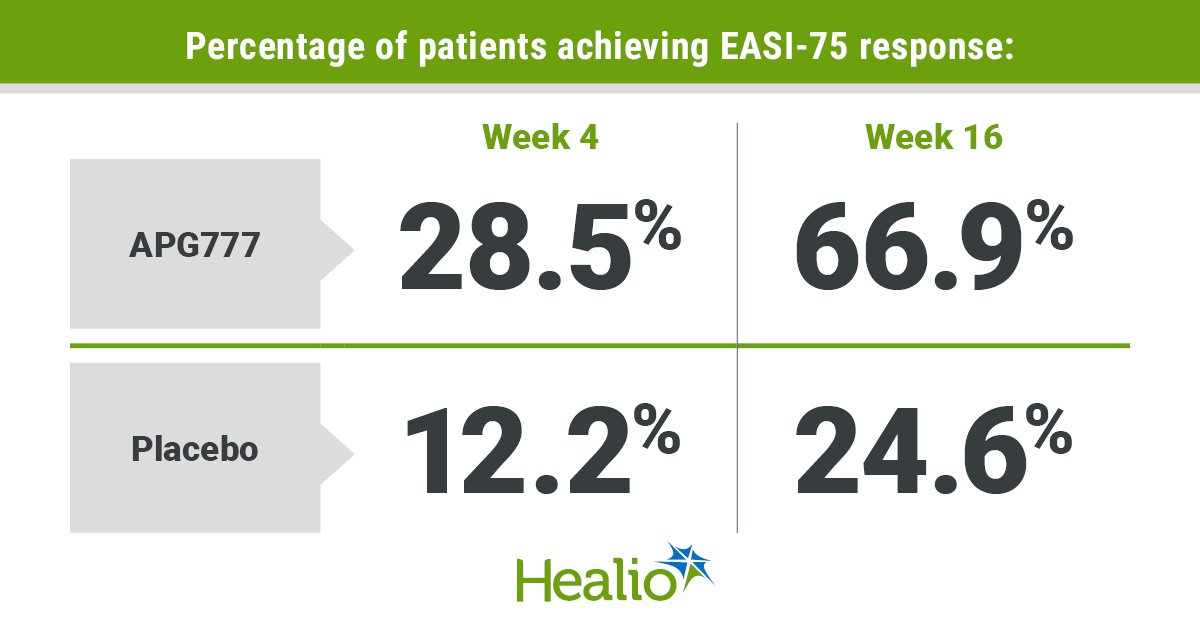
Additionally, percentages of sufferers who achieved a 75% discount or higher of their EASI scores (EASI-75) at week 4 included 28.5% of the therapy group and 12.2% of the placebo group (P < .05). At week 16, these percentages grew to 66.9% of the therapy group and 24.6% of the placebo group (P < .001).
“That’s the measure that about 70% of physicians search for to determine if they will prescribe a biologic to an atopic derm affected person,” Henderson stated. “Simply over two out of each three sufferers reply on that metric.”
Apogee Therapeutics referred to as this highest absolute and placebo-adjusted EASI-75 of any biologic at week 16, reporting 49.5% for dupilumab, 53% for lebrikizumab-lbks, and 29.1% for tralokinumab-ldrm (Adbry, LEO Pharma).
“We will, sooner or later, take into consideration going out to medical doctors and physicians and saying, ‘Look, customary of care is one out of two sufferers will reply,’” Henderson stated. “What about two out of three sufferers responding? And also you’re giving that injection each 3 months or much less.”
Stratified by publicity, 83.3% of quartile three and 89.5% of quartile 4 achieved EASI-75 responses with APG777 as effectively.
In the meantime, 33.9% of the therapy group and 14.7% of the placebo group achieved a 90% discount or higher of their EASI scores (P < .05), with 63.2% of the quartile with the best publicity reaching EASI-90 responses.
By comparability, 31.8% of sufferers on dupilumab, 34.5% of these on lebrikizumab-lbks, and 16.4% of these on tralokinumab-ldrm achieved EASI-90 responses.
Validated Investigator International Assessments included 34.9% of the therapy group and 17.3% of the placebo group reaching a rating of 0 or 1 (P < .05), with 63.2% of the quartile with the best publicity reaching a rating of 0 or 1.
Apogee Therapeutics stated these findings have been in line with the usual of care, together with 34.6% for dupilumab, 37% for lebrikizumab-lbks and 19% for tralokinumab-ldrm.
Additional, LSM % modifications from baseline in Itch Numerical Score Scale (NRS) scores included 50.7% for the therapy group and 23.2% for the placebo group at week 16, Apogee Therapeutics stated. By comparability, figures included 45.1% for dupilumab and 41.1% for lebrikizumab-lbks.
“Itch is admittedly essential. We’re seeing vital discount in itch as early as 1 week,” Henderson stated, including that it produced “deeper itch decision than any biologic alone has additionally achieved.”
Nemolizumab-ilto (Nemluvio, Galderma) yielded a 55.9% LS imply % change from baseline in Itch NRS, the researchers famous.
“However that’s solely together with topical corticosteroids,” Henderson stated. “When it comes to finest biologic, not with [topical corticosteroids] included, we’re seeing the perfect knowledge as effectively in itch.”
Security profile
The corporate additionally categorized APG777’s security profile as in keeping with different brokers in its class, with 1.2% of the therapy group and a pair of.4% of the placebo group experiencing severe treatment-emergent adversarial occasions. Additionally, 2.4% of the therapy group discontinued therapy attributable to adversarial occasions.
“These are very protected biologics and mechanisms,” Henderson stated.
The commonest treatment-emergent adversarial occasions included noninfective conjunctivitis, impacting 14.6% of the therapy group and a pair of.4% of the placebo group; higher respiratory tract infections, impacting 8.5% of the therapy group and 12.2% of the placebo group; and nasopharyngitis, impacting 4.9% of the therapy group and 12.2% of the placebo group.
“The one adversarial occasion that’s shared among the many class is you get a little bit of purple eyes —conjunctivitis,” Henderson stated. “It is a benign and transient occasion, and it’s solely seen in atopic derm.”
When these biologics are used for different indications, he defined, conjunctivitis doesn’t happen.
“It’s one thing peculiar to the biology, and it goes down as you enhance exposures,” Henderson stated. “Some docs have advised us it’s an indication that you simply’re hitting the pathway.”
Additionally, the therapy group had no injection web site reactions.
“You have a look at previous trials and the actual world. Lots of people simply proceed Dupixent. It’s an excellent drug. But it surely hurts. It stings,” Henderson stated. “Our complete factor is a greater affected person and doctor expertise.”
Subsequent steps
Outcomes from the upkeep section of half A by 3 months and 6 months are anticipated within the first half of 2026, the corporate stated.
Half B will comprise 280 sufferers receiving low, medium or excessive doses of APG777 or placebo, with the excessive dose approximating twice the publicity of lebrikizumab. Outcomes are anticipated in mid-2026.
“We’ve designed half B to actually be the right experiment, the complete vary of dosing to see how we will determine what’s the finest dose to take ahead for atopic derm sufferers,” Henderson stated.
As soon as the half B knowledge is obtainable, Henderson stated, Apogee will transfer into section 3 research subsequent yr with a objective of FDA approval and business launch in 2029.
“We’re very thrilled with the information, and I’ve had very nice remarks from our physicians and sufferers that we’ve spoken with who simply suppose that this might remodel care,” Henderson stated. “It’s simply actually thrilling.”
Reference:
For extra info:
Michael Henderson, MD, might be reached at allergy@healio.com.