Key takeaways:
- Fremanezumab is a human monoclonal antibody that targets calcitonin gene-related peptides.
- Therapy doses various by weight.
- The security profiles for the remedy and placebo teams had been comparable.
MINNEAPOLIS — Pediatric sufferers with fewer than 15 headache days every month noticed higher outcomes with fremanezumab in contrast with placebo, in accordance with a speaker on the American Headache Society 67th Annual Scientific Assembly.
Complications are episodic options of migraine, which is a illness, not an occasion, Andrew D. Hershey, MD, PhD, FAAN, FAHS, endowed chair and director of neurology, Cincinnati Kids’s Hospital Medical Heart, stated throughout his presentation.

Information had been derived from Hershey AD, et al. Efficacy and security of fremanezumab for the preventive remedy of episodic migraine in kids and adolescents: A part 3, randomized, double-blind, placebo-controlled examine. Introduced at: American Headache Society 67th Annual Assembly; June 19-22, 2025; Minneapolis.
“We’ve created this false dichotomy of episodic and power complications, when it’s actually simply complications attributable to migraine,” Hershey stated.
Hershey additionally disagreed with classifications resembling pediatric or adolescent migraine, including that physicians can draw from their expertise in treating adults to handle youthful sufferers.
“It’s one illness throughout the lifespan,” he stated. “The sooner we will intervene within the illness, the extra probably we’re to have an effect on the outcomes.”
The FDA permitted fremanezumab (Ajovy, Teva Prescribed drugs), a humanized monoclonal antibody that targets calcitonin gene-related peptides (CGRPs), for migraine in adults in 2018.
The SPACE examine examined its use amongst 237 pediatric sufferers (55% women, 77% white), together with 64 kids aged 6 to 11 years and 171 adolescents aged 12 to 17 years.
“Not like the grownup research, the place it’s largely feminine, we had a fairly even distribution of male to feminine in children that we see,” Hershey stated.
Month-to-month doses within the remedy group included 120 mg for 36 sufferers who weighed fewer than 45 kg (median age, 11 years) and 225 mg for 87 sufferers who weighed 45 kg or extra (median age, 15 years), with 112 receiving placebo (median age, 14 years).
“A migraine day, simply by definition, is a headache day by which sufficient of the options had been in line with a migraine,” Hershey stated. “Once more, migraine is the illness. Headache is the symptom.”
At baseline, he continued, a lot of the sufferers within the examine had been experiencing between 7.5 and eight migraine days every month.
“A couple of third of the month, they had been having a headache,” he stated.
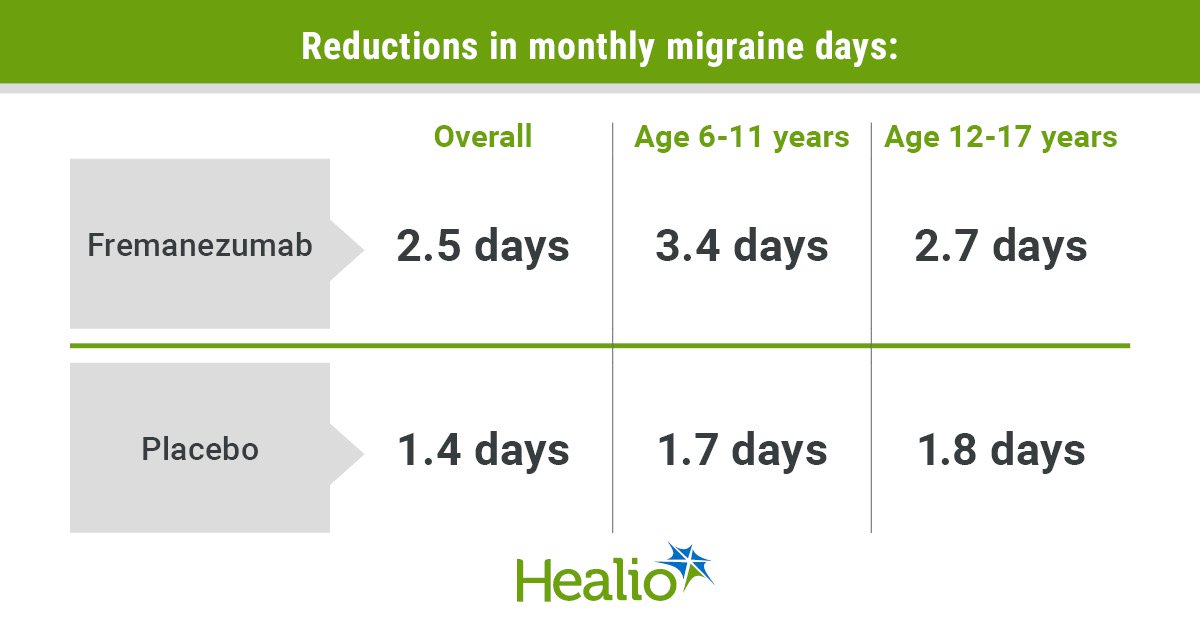
Whole migraine days every month fell by 2.5 days for the remedy group and by 1.4 days for the placebo group (P = .021) from baseline by means of 3 months. Though “there was not a statistical separation” between the 120 mg and the 225 mg group, Hershey stated, the 225 mg group skilled “extra dramatic” enhancements.
Among the many youthful kids, whole migraine days fell by 3.4 days within the remedy group and by 1.8 days within the placebo group. Amongst adolescents, whole migraine days fell by 2.7 days for the remedy group and by 1.8 days for the placebo group.
Further decreases included 3.5 days for the remedy group and a couple of.2 days for the placebo group amongst boys, in addition to 2.3 days for the remedy group and 1.5 days for the placebo group amongst women.
Decreases in month-to-month headache days included 2.6 for the remedy group and 1.5 for the placebo group (P = .0172). Additionally, 47.2% of the remedy group and 27% of the placebo group (P = .0016) noticed a 50% discount or higher in month-to-month migraine days.
General, “everybody received higher, even the placebo arm,” Hershey stated, “which is finally what we would like.”
Percentages of sufferers who skilled a minimum of one antagonistic occasion included 49% of the placebo group, 56% of the 120 mg group, 55% of the 225 mg group, and 55% of the remedy group general.
Equally, percentages of sufferers who skilled a minimum of one severe antagonistic occasion included 3% of the placebo group, 3% of the 120 mg group, 1% of the 225 mg group, and a couple of% of the remedy group general.
The most typical antagonistic occasions occurring in 5% or extra of sufferers included infections and infestations, affecting 28% of the placebo group, 25% of the 120 mg group, 28% of the 225 mg group, and 27% of the remedy group general.
Subsequent, basic issues and administration website situations affected 19% of the placebo group, 14% of the 120 mg group, 24% of the 225 mg group and 21% of the remedy group general.
“These had been all very well-tolerated,” Hershey stated, including that this profile was in line with what has been seen elsewhere in remedy with anti-CGRP monoclonal antibodies. “Nothing actually surprises us.”
Based mostly on these findings, the researchers concluded that kids and adolescents with fewer than 15 headache days monthly can see important enhancements with fremanezumab.
Hershey additionally stated that this examine was one of many first to reveal fremanezumab’s efficacy in contrast with placebo on this age group.
“It reveals children can do research,” he stated. “Youngsters can do research nicely, and we will get good outcomes.”
The researchers are actually compiling knowledge on the usage of fremanezumab amongst kids and adolescents with greater than 15 headache days every month.
“These outcomes might be popping out very quickly,” he stated.
For extra info:
Andrew D. Hershey, MD, PhD, FAAN, FAHS, might be reached at neurology@healio.com.