Key takeaways:
- The mRNA shot stacked up effectively towards a licensed standard-dose influenza vaccine.
- The corporate stated it plans to interact with regulators to file submissions for approval.
Moderna this week introduced constructive information from a part 3 trial of its messenger RNA vaccine towards seasonal influenza, which may set the stage for the corporate’s mixture COVID-19/influenza shot.
“The severity of this previous flu season underscores the necessity for simpler vaccines,” Moderna CEO Stéphane Bancel, MSc, MBA, stated in a press launch. “An mRNA-based flu vaccine has the potential benefit to extra exactly match circulating strains, help fast responses in a future influenza pandemic and pave the way in which for COVID-19 mixture vaccines.”

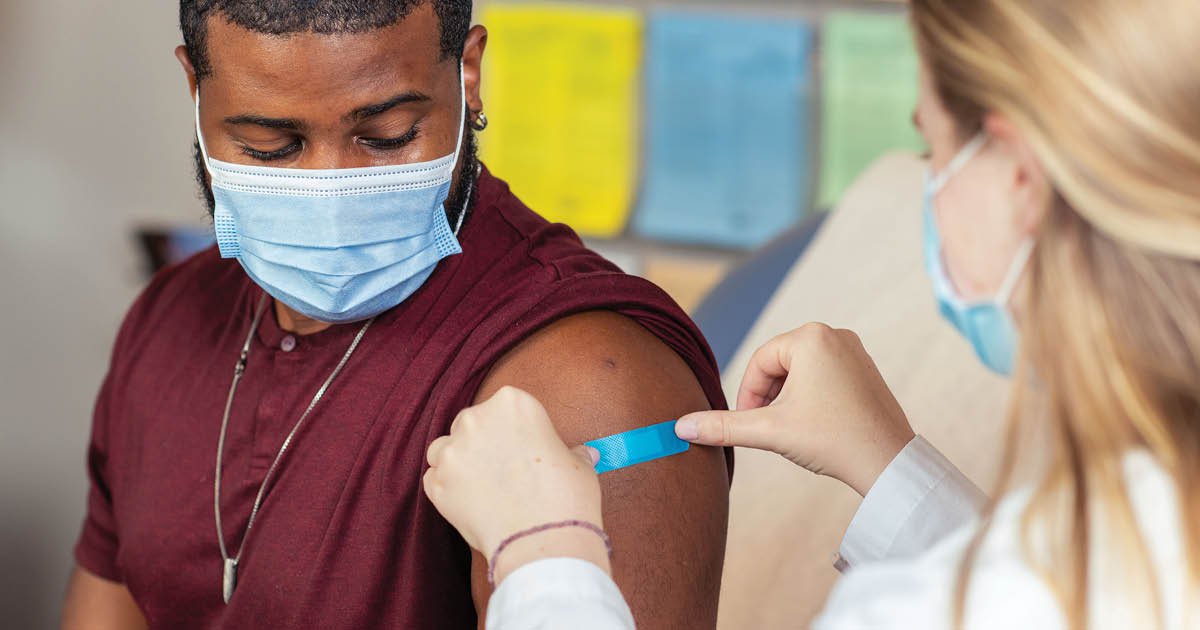
Moderna reported constructive information from a part 3 efficacy research of its mRNA-based seasonal influenza vaccine. Picture: CDC/Brandon Clifton
In Might, Moderna withdrew its FDA submission for the mix COVID-19/influenza vaccine, mRNA-1083, and stated it could resubmit the applying later this yr after efficacy information have been accessible from the trial of the influenza vaccine. The corporate reported constructive information on the mix shot in 2023.
Optimistic outcomes
The present trial enrolled almost 41,000 adults aged 50 years or older at 301 websites in 11 nations and randomly assigned them to obtain both a single dose of the mRNA influenza vaccine, mRNA-1010, or a standard-dose of a comparable licensed vaccine. Researchers adopted contributors for a median of 6 months.
In response to outcomes reported by Moderna, the relative vaccine efficacy (rVE) of mRNA-1010 was 26.6% (95% CI, 16.7%-35.4%) greater than the licensed vaccine amongst all contributors, and a bit greater than that amongst contributors aged 65 years or older.
The rVE was excessive towards the three influenza viruses focused by present seasonal vaccines — A/H1N1 (29.6%), A/H3N2 (22.2%) and B/Victoria (29.1%).
In response to Moderna, the protection and tolerability of mRNA-1010 within the part 3 trial was much like a earlier part 3 research of the vaccine, with injection website ache as the most typical native solicited antagonistic response and fatigue, headache and myalgia as the most typical systemic reactions. Most of those reactions, the corporate stated, have been gentle.
Moderna plans to current the information at an upcoming medical convention, publish the findings in a peer-reviewed publication and work with regulators on submitting functions for FDA approval.
Unsure panorama
The panorama for mRNA vaccines seems to be totally different than it did when Moderna initiated the trial. HHS Secretary Robert F. Kennedy Jr. and different high well being officers have been crucial of COVID-19 mRNA vaccines, and a majority of the new members of the CDC’s Advisory Committee on Immunization Practices (ACIP) — which makes federal suggestions for accepted vaccines — have publicly doubted the protection or effectiveness of these pictures regardless of heaps of proof displaying they’re each secure and efficient.
That features the committee’s new chair, Martin Kulldorff, PhD, who wrote in Metropolis Journal final yr that the trials for the COVID-19 vaccines “weren’t correctly designed,” and Robert Malone, MD, who performed early analysis on mRNA vaccines however grew to become a vocal opponent of them through the COVID-19 pandemic.
One other new member, Vicky Pebsworth, OP, PhD, RN, is the director of analysis and affected person security on the Nationwide Vaccine Data Middle, which stated final month that COVID-19 mRNA vaccines shouldn’t be beneficial for anybody. A fourth ACIP member, Retsef Levi, PhD, professor on the MIT Sloan College of Administration, as soon as publicly referred to as for mRNA vaccine administration to be stopped based mostly on unsubstantiated proof that the pictures “trigger critical hurt together with dying.”
Amesh A. Adalja, MD, senior scholar on the Johns Hopkins Middle for Well being Safety, expressed concern that the ACIP will ignore information for private biases when making vaccine suggestions.
“I’m involved about how ACIP will deal with any resolution, not to mention ones regarding mRNA vaccines,” he instructed Healio. “The ACIP, as presently constituted, is an anti-vaccine group stacked with individuals who evade the worth of vaccines, and the mRNA vaccines are a specific anathema to their irrational minds. I totally anticipate the ACIP making all the vaccine ecosystem hostile to vaccine applied sciences, particularly mRNA, to the detriment of human well being.”
References:
- CDC. Flu burden: Preliminary estimated flu illness burden 2024-2025 flu season. https://www.cdc.gov/flu-burden/php/data-vis/2024-2025.html. Printed Might 9, 2025. Accessed July 1, 2025.
- ClinicalTrials.gov. A research of mRNA-101 in contrast with a licensed influenza vaccine in adults 50 years of age. https://clinicaltrials.gov/research/NCT06602024. Up to date Might 14, 2025. Accessed July 1, 2025.
- Kulldorff M. Harvard tramples the reality. Metropolis Journal. https://www.city-journal.org/article/harvard-tramples-the-truth. Printed March 11, 2024. Accessed June 12, 2025.
- Moderna proclaims constructive part 3 outcomes for seasonal influenza vaccine. https://buyers.modernatx.com/information/news-details/2025/Moderna-Broadcasts-Optimistic-Part-3-Outcomes-for-Seasonal-Influenza-Vaccine/default.aspx. Printed June 30, 2025. Accessed July 1, 2025.
- Moderna supplies replace on BLA submission for mixture vaccine towards influenza and COVID-19. https://information.modernatx.com/information/news-details/2025/Moderna-Gives-Replace-on-BLA-Submission-for-Mixture-Vaccine-In opposition to-Influenza-and-COVID-19/default.aspx. Printed Might 21, 2025. Accessed July 1, 2025.
- Nationwide Vaccine Data Middle. NVIC to FDA: mRNA COVID-19 pictures shouldn’t be beneficial for anybody. https://www.nvic.org/e-newsletter/june-2025/public-comment-nvic-may-2025. Printed June 13, 2025. Accessed July 2, 2025.
- Retsef Levi. (@RetsefL). Jan. 30, 2023. X.
For extra data:
Amesh A. Adalja, MD, will be contacted at infectiousdisease@healio.com.