A drug used for Parkinson’s illness has been proven to be efficient in decreasing the signs of adverse to deal with melancholy, based on a research led by the College of Oxford.
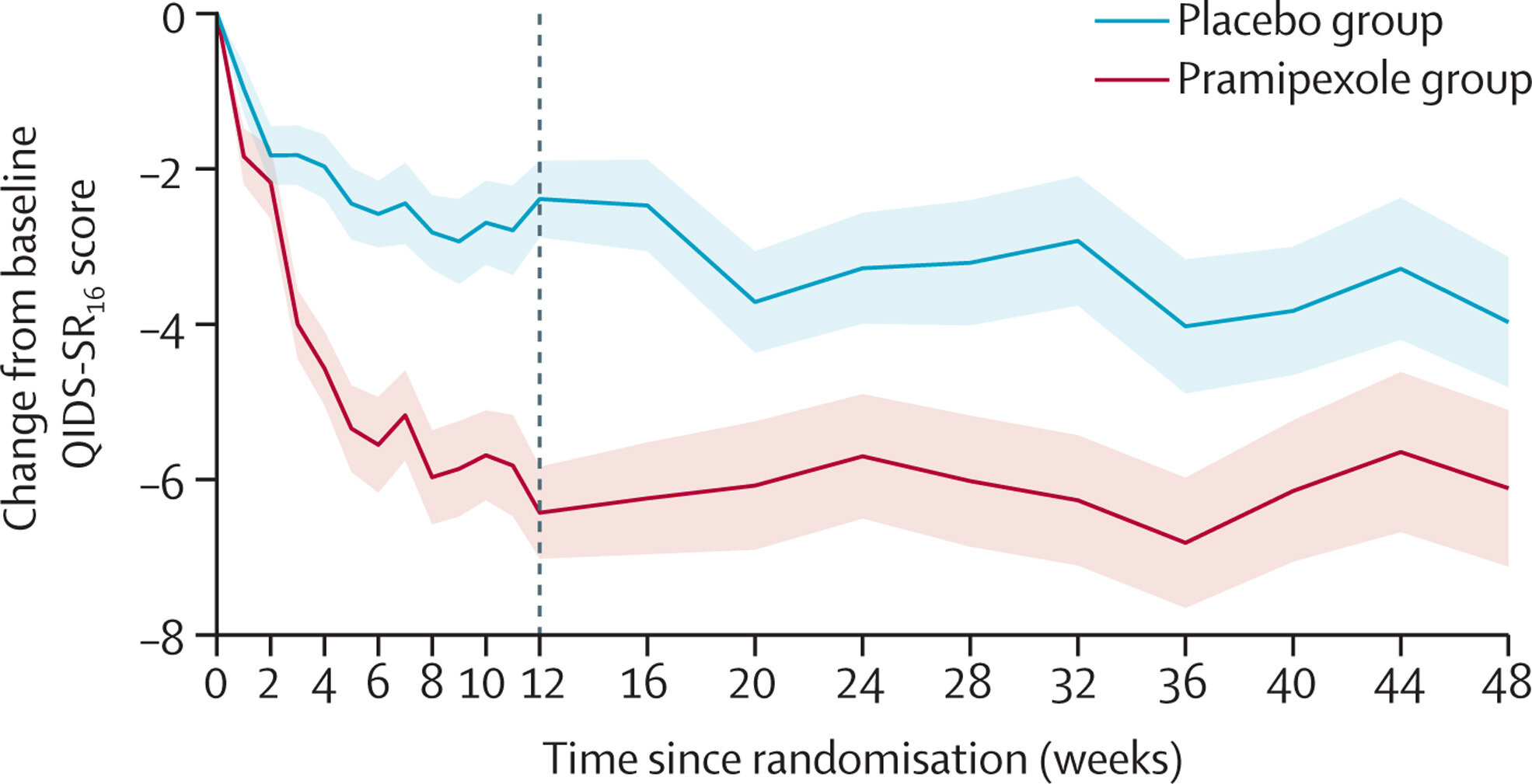
Within the largest medical trial to this point, pramipexole was discovered to be considerably more practical than a placebo at decreasing the signs of treatment-resistant melancholy (TRD) over the course of practically a 12 months, when added to ongoing antidepressant remedy.
The trial, revealed in The Lancet Psychiatry, included 150 sufferers with treatment-resistant melancholy, with equal numbers receiving 48 weeks of pramipexole or a placebo, alongside ongoing antidepressant remedy.
General, the group taking pramipexole skilled a major and substantial discount in signs by week 12 of therapy, with the advantages persisting over the course of a 12 months. Nonetheless, there have been additionally important unwanted effects, corresponding to nausea, sleep disturbance and dizziness, with about 1 in 5 individuals on pramipexole dropping out of the trial consequently.
Professor Michael Browning, from the Division of Psychiatry, College of Oxford, and workstream lead in Temper Problems for the NIHR Psychological Well being-Translational Analysis Collaboration (MH-TRC) Mission, who led the trial, mentioned, “Successfully treating individuals who haven’t responded to first-line interventions for melancholy is a urgent medical downside and there has lengthy been an pressing want to seek out new therapies.
“These findings on pramipexole are a major breakthrough for sufferers for whom antidepressants and different therapies and therapies haven’t labored.
“Pramipexole is a medication licensed for Parkinson’s illness and works by boosting the mind chemical dopamine. This differs from nearly all of different antidepressant medicines which act on mind serotonin and will clarify why pramipexole was so useful on this research.
“We now want extra analysis specializing in decreasing the unwanted effects of pramipexole, evaluating its cost-effectiveness, and evaluating it with different add-on therapies.”
Earlier analysis into utilizing the drug for melancholy had proven promise, however there had been restricted information on its long-term outcomes and unwanted effects till now.
Present pointers for individuals with treatment-resistant melancholy advocate including new therapies, corresponding to lithium or antipsychotics, to ongoing antidepressant therapy, however these have restricted effectiveness and don’t work for everybody.
Phil Harvey, 72, from Oxfordshire, was recognized with melancholy 20 years in the past and tried completely different tablets and counseling however nothing labored. Ultimately he needed to take a 12 months off work earlier than retiring. He began on the trial in 2022.
He mentioned, “Inside a couple of weeks I felt the results, it was superb. I saved a diary which they gave us on how my temper was, motivation and the way it improved. It was dragging me out of this darkish black gap that I have been in for years.”
Individuals had been recruited from throughout the nation, together with as a part of the MH-TRC Mission temper dysfunction clinics, that are hosted at Oxford however positioned throughout the nation. The clinics effectively, and largely remotely, assess sufferers with difficult-to-treat temper problems and provide them enrollment in analysis research. The community can even help major care companies by offering evaluation and therapy recommendation for sufferers who haven’t responded to preliminary therapy.
Extra info:
Michael Browning et al, Pramipexole augmentation for the acute part of treatment-resistant, unipolar melancholy: a placebo-controlled, double-blind, randomised trial within the UK, The Lancet Psychiatry (2025). DOI: 10.1016/S2215-0366(25)00194-4. www.thelancet.com/journals/lan … (25)00194-4/fulltext
Quotation:
Parkinson’s drug reduces signs in treatment-resistant melancholy, medical trial finds (2025, June 30)
retrieved 30 June 2025
from https://medicalxpress.com/information/2025-06-parkinson-drug-symptoms-treatment-resistant.html
This doc is topic to copyright. Aside from any truthful dealing for the aim of personal research or analysis, no
half could also be reproduced with out the written permission. The content material is supplied for info functions solely.