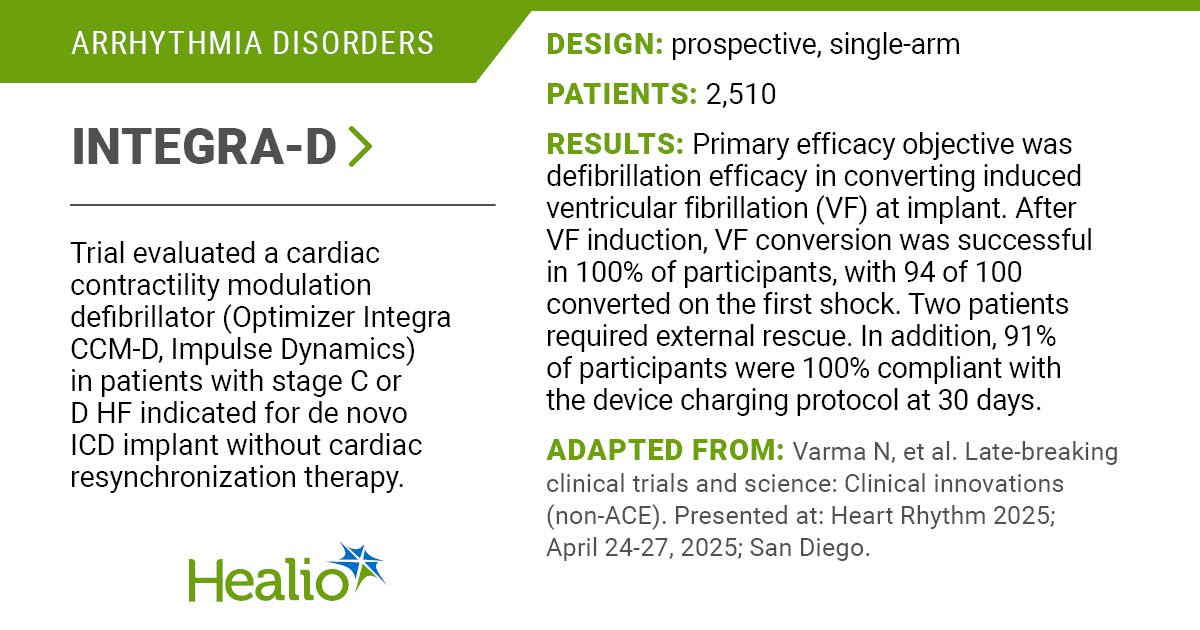
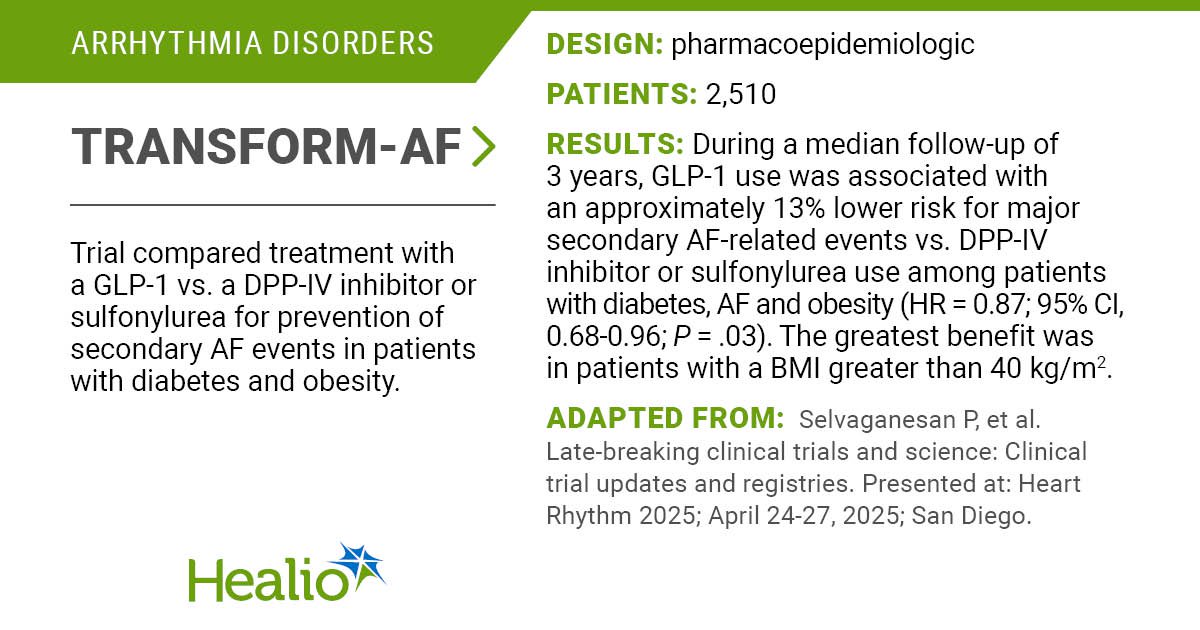
The PULSAR IDE trial evaluated a spherical, all-in-one pulsed discipline ablation catheter for sufferers with paroxysmal atrial fibrillation ablation.
Learn Healio’s in-depth protection of the PULSAR IDE trial.

The trial enrolled 183 sufferers present process catheter ablation for paroxysmal AF at 12 websites within the U.S., Canada and Europe. Nineteen operators carried out the ablation procedures.
The all-in-one pulsed discipline ablation catheter system (Globe PF System, Kardium) demonstrated sturdy pulmonary vein isolation and promising security, with no device-related occasions at 1 yr.
Complete time from the primary to final pulsed discipline ablation software was roughly 26 minutes and the typical variety of PVI purposes was 1.2.
Outcomes of the PULSAR IDE trial had been introduced at Coronary heart Rhythm 2025.