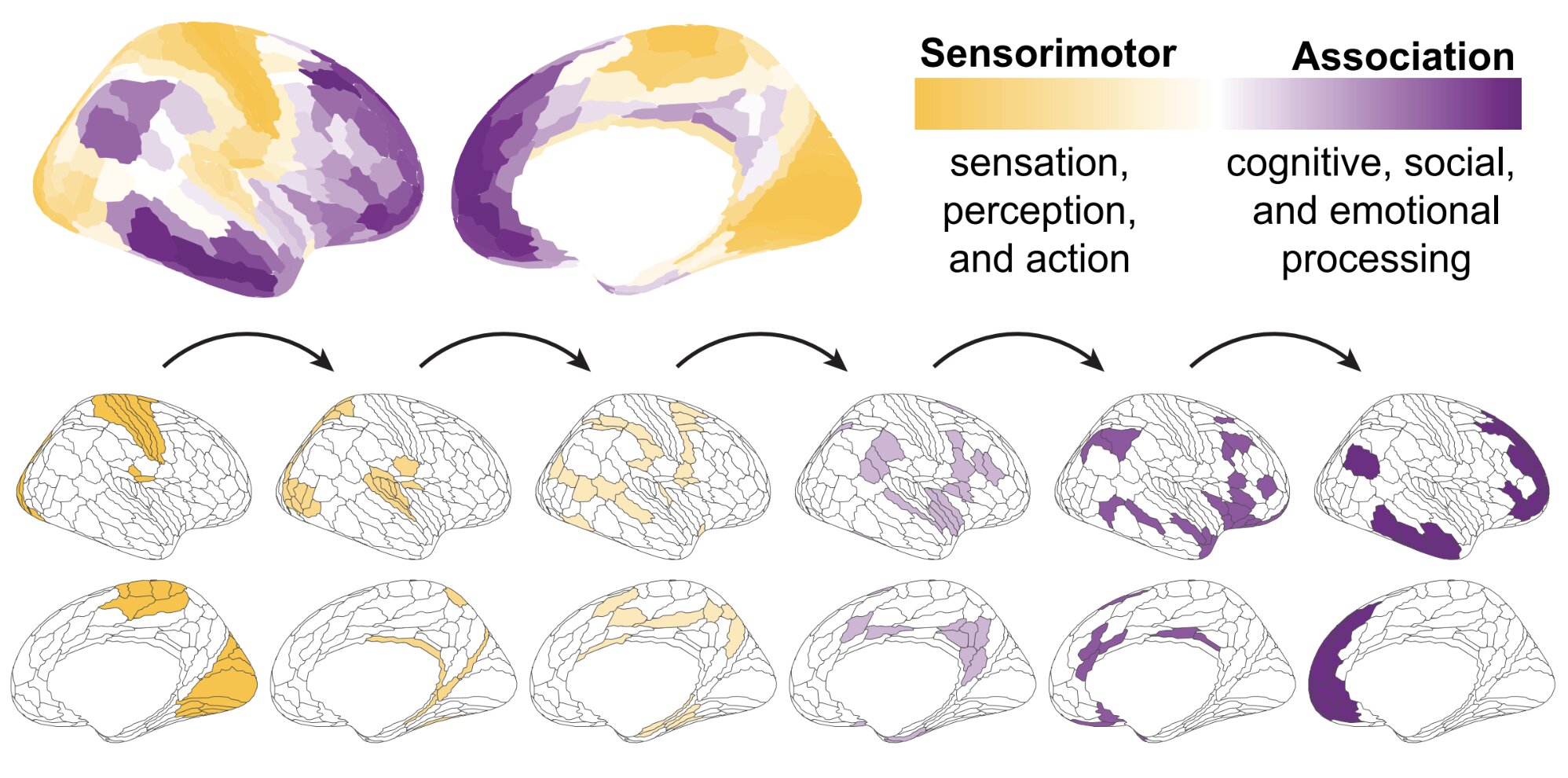
Guselkumab, whether or not administered each 4 or 8 weeks, demonstrates “important inhibition” of structural injury in sufferers with energetic psoriatic arthritis, in response to information offered on the EULAR 2025 Congress.
“The target of this part 3b examine is to extra intently consider the flexibility of each This fall- and Q8-week dosing to inhibit structural injury development,” Philip J. Mease, MD, of the Windfall Swedish Medical Heart, in Seattle, advised attendees.

Mease described guselkumab (Tremfya, Janssen) as a completely human monoclonal antibody that selectively targets the interleukin (IL)-23p19 subunit, which can inhibit IL-23 signaling.
“It’s twin appearing,” he stated. “That implies that it not solely binds to IL-23, but it surely additionally binds to the CD64 receptor on IL-23, producing myeloid cells. It’s there within the room the place the motion is.”
Within the APEX trial, a randomized, double-blind, placebo-controlled examine, Mease and colleagues outlined energetic PsA as three or extra tender and swollen joints, elevated C-reactive protein and two or extra joints with radiographic erosion regardless of prior therapeutic intervention.
The evaluation included 273 sufferers handled with guselkumab 100 mg each 4 weeks; 371 sufferers handled with guselkumab 100 mg at weeks 0 and 4, after which each 8 weeks thereafter; or placebo each 4 weeks.
The proportion of sufferers attaining ACR20 respose at 24 weeks served as the first endpoint. A co-primary endpoint of imply change from baseline in PsA modified van der Heijde-Sharp (vdH-S) rating at week 24 additionally was additionally assessed.
In accordance with the researchers, 67% of sufferers who obtained guselkumab 100 mg each 4 weeks, and 68% of those that obtained guselkumab 100 mg each 8 weeks, achieved the ACR20 endpoint at 24 weeks, in contrast with 47% of sufferers within the placebo group (P < .001).
“As you’ll be able to see, the [primary endpoint] was met,” Mease stated.
The guselkumab arms moreover demonstrated much less radiographic development, in response to the researchers. The 4-week group confirmed a least squares imply change in PsA-modified vdH-S rating of 0.55 (P = .002 vs. placebo), in contrast with 0.54 within the 8-week group (P < .001 vs. placebo) and 1.35 amongst those that obtained placebo.
“As you’ll be able to see, in each dose arms, whether or not the affected person was receiving guselkumab each 8 weeks or each 4 weeks, there was important inhibition of structural injury development as in contrast with placebo,” Mease stated. “That is the important thing slide of the presentation.”
Modifications in joint area narrowing and erosion scores adopted comparable traits, as did the proportions of sufferers with no radiographic development, he added.
Adversarial occasions occurred in 38% and 42% of the This fall- and Q8-week therapy teams, respectively, in contrast with 37% amongst those that obtained placebo. Critical adversarial occasion charges had been 3% or decrease for all teams. The researchers recognized no new security indicators.
“This examine met its main endpoint of an ACR20 response vs. placebo in each dose arms of guselkumab,” Mease stated. “There have been considerably decrease charges of radiographic development in each dose arms.”