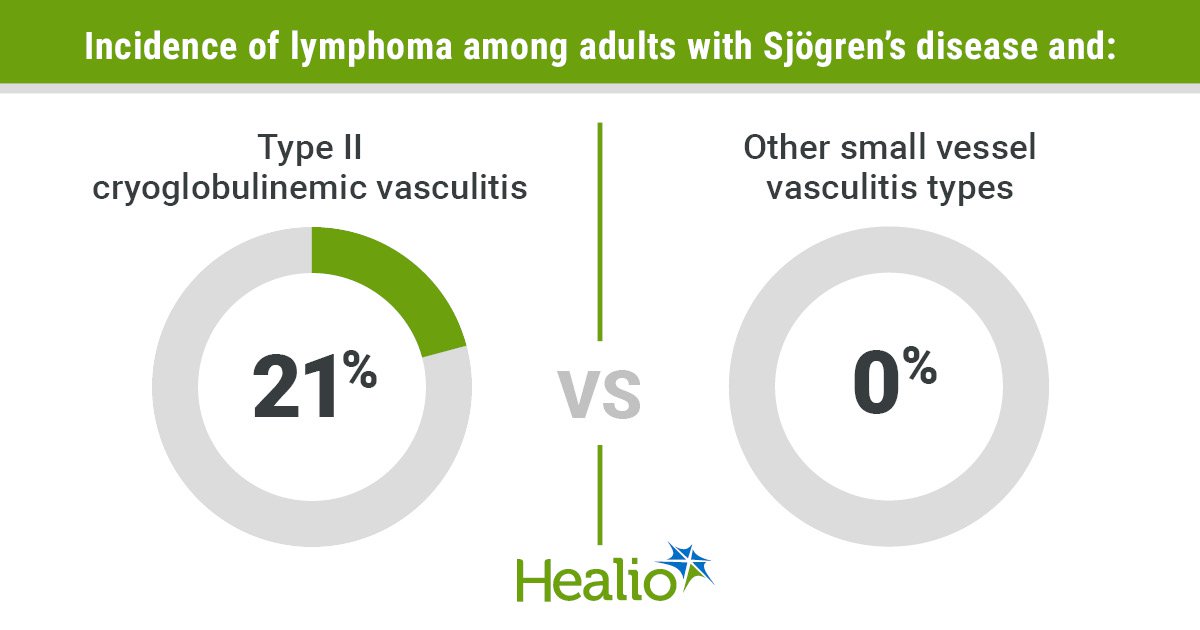
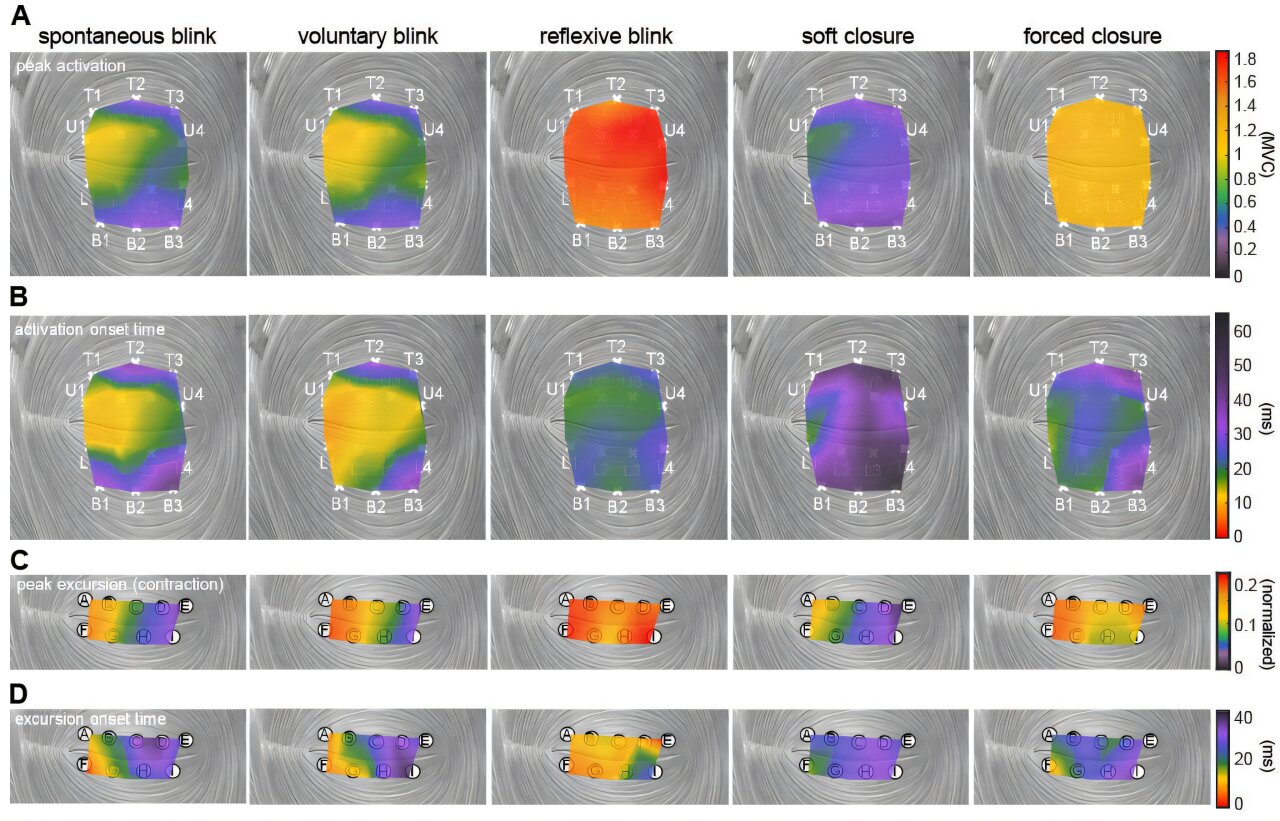
Whats up, everybody, and the way are you at the moment? We’re doing simply wonderful, thanks, particularly because the center of the week is already upon us. In any case, we have now made it this far — regardless of the impressively excessive temperatures — so we have now determined to hold on for one more couple of days. And why not? Given the doubtless alternate options, this appears to be an affordable resolution. To make the time fly, we’re firing up the trusted espresso kettle and brewing one other cup of stimulation. Our alternative at the moment is pistachio creme, which occupies a distinguished spot in our pantry. However now, the time has come to get cracking. Listed below are just a few gadgets of curiosity that will help you get began. We hope you have got a stunning day, and do be in contact. Suggestions, ideas, and strategies are all the time welcome. …
The U.S. Meals and Drug Administration is investigating studies of two deaths attributable to acute liver failure in non-ambulatory Duchenne muscular dystrophy sufferers after receiving a Sarepta Therapeutics gene remedy referred to as Elevidys, Reuters notes. Earlier this month, Sarepta reported a second demise in a affected person who had obtained its gene remedy, which raised issues in regards to the security and future demand for the therapy. The sufferers who died have been a 16-year-old, weighing 154 kilos, and a 15-year-old, weighing 110 kilos. Each boys have been non-ambulatory and their deaths occurred inside 90 days after therapy, the corporate instructed analysts final week in an investor name. The 2 sufferers confirmed indicators of acute liver failure and have been hospitalized lower than two months after therapy with Elevidys, in line with the FDA, which added that it’s evaluating the necessity for additional regulatory motion. The U.S. prescribing info for Elevidys warns of acute liver harm however doesn’t point out liver failure or demise. Elevidys, accredited by the FDA in 2024 for ambulatory Duchenne muscular dystrophy sufferers aged 4 and older, is the one gene remedy out there for the illness.
In a bid to transform the controversial relationship between drugmakers and affected person charities, Regeneron Prescribed drugs has introduced a brand new program through which it is going to match as much as $200 million in donations this 12 months to a number one basis referred to as Good Days, STAT explains. The transfer comes after Regeneron a number of months in the past slashed its funding to the charity, an sudden step that appeared largely designed to blunt the usage of eye medicines bought by rivals, however one which has decreased affected person entry and, in flip, sparked concern amongst buyers and physicians. Certainly, that call has slowed the corporate’s income development and has prompted some ophthalmologists to complain their sufferers have been shedding entry to wanted remedies. The event displays the aggressive calculus of the multibillion-dollar marketplace for costly eye medicines that threatens to get more and more crowded, particularly now {that a} biosimilar model of a Regeneron drug is on the market. The Regeneron transfer additionally seems rooted in ongoing challenges that drugmakers face when bankrolling charities in ways in which have drawn scrutiny from U.S. authorities officers.


This text is unique to STAT+ subscribers
Unlock this text — plus in-depth evaluation, newsletters, premium occasions, and information alerts.
Have already got an account? Log in