Key takeaways:
- Dupixent beforehand acquired precedence overview and orphan drug designation from the FDA for bullous pemphigoid.
- This approval is supported by information from the part 2/3 ADEPT trial.
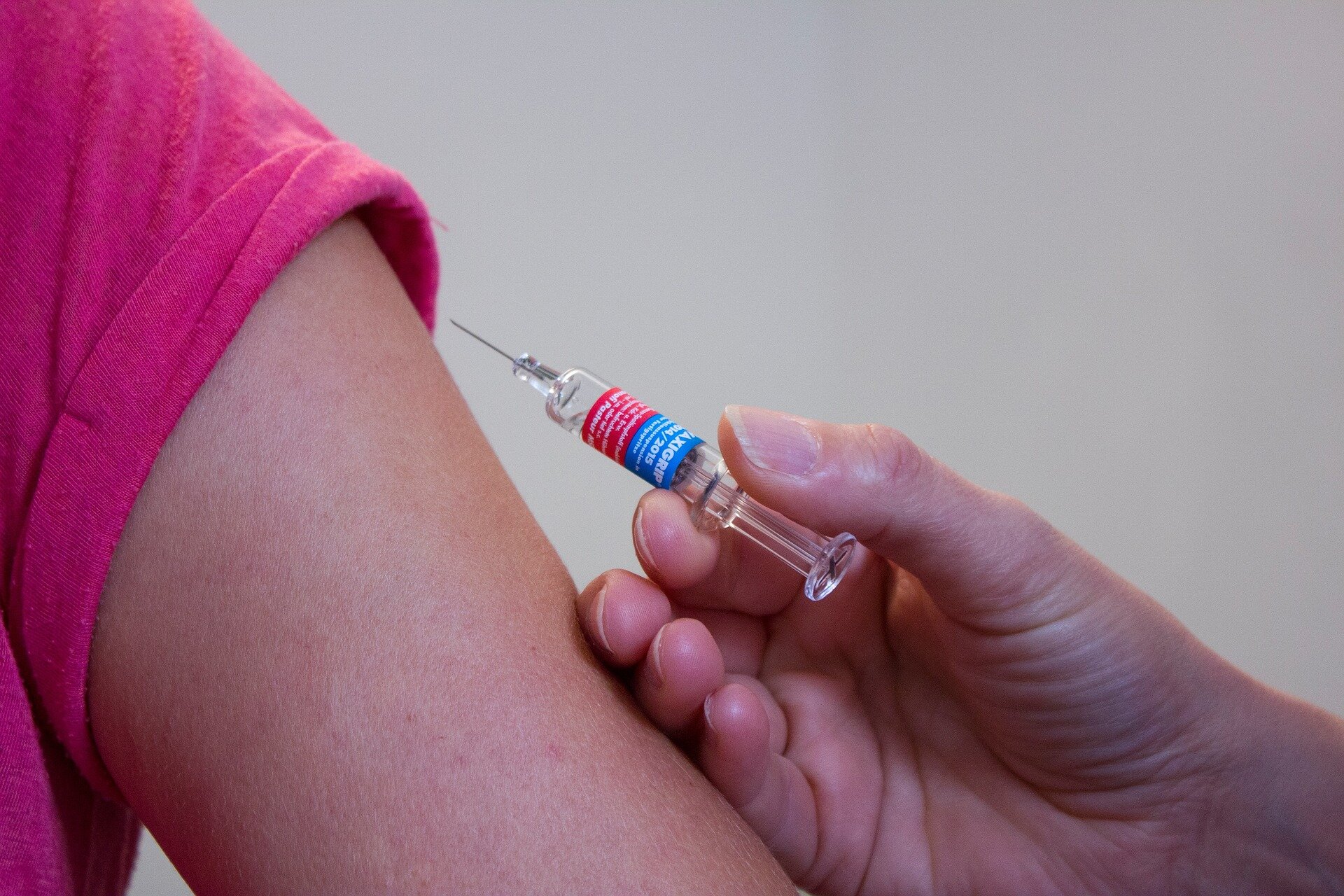
The FDA permitted Dupixent for the therapy of adults with bullous pemphigoid, a uncommon pores and skin illness usually uncontrolled with systemic corticosteroids, Sanofi and Regeneron introduced.
The FDA’s determination makes Dupixent (dupilumab), an interleukin-4 and -13 inhibitor, the primary permitted therapy indicated for bullous pemphigoid (BP) in the USA. The approval marks the drug’s eighth indication for ailments with underlying sort 2 irritation.

The FDA permitted Dupixent for the therapy of adults with bullous pemphigoid.
“BP is a persistent, debilitating and relapsing uncommon pores and skin illness that may trigger intense itching, and painful blisters and lesions, and primarily impacts aged sufferers,” Donna Culton, MD, PhD, a lead investigator of the ADEPT trial and professor of dermatology at College of North Carolina Chapel Hill, advised Healio. “Beforehand, accessible therapy choices had been restricted and infrequently concerned corticosteroids and immunosuppressants — medicines that added to affected person burden attributable to suppression of the immune system, frequent lab monitoring and critical uncomfortable side effects.”

Donna Culton
Dupilumab is protected and efficient for the therapy of BP, in accordance with information from the ADEPT part 2/3 examine.
As Healio beforehand reported, researchers randomly assigned adults with average to extreme BP to obtain dupilumab 300 mg (n = 53) or placebo (n = 53), in addition to a routine of standard-of-care oral corticosteroids. Those that achieved illness management tapered off their oral corticosteroids.
Outcomes confirmed that after 36 weeks of therapy, 18.3% of sufferers handled with dupilumab skilled sustained illness remission vs. 6.1% of these handled with placebo. A better proportion of individuals handled with dupilumab additionally achieved a clinically significant itch discount in contrast with the placebo group (38.3% vs. 10.5%).
Within the dupilumab group, the median cumulative oral corticosteroid dose was 2.8 g whereas the dose amongst these receiving the placebo was 4.1 g. Adversarial occasions amongst these handled with dupilumab vs. placebo included arthralgia, conjunctivitis, blurred imaginative and prescient, herpes viral infections and keratitis. A case of acute generalized exanthematous pustulosis was additionally reported.
The FDA beforehand granted precedence overview and orphan drug designation to dupilumab for BP due to the illness’s uncommon and critical nature, affecting fewer than 200,000 folks within the U.S. This approval marks a long-awaited victory for sufferers and their suppliers, in accordance with Culton.
“With the approval of Dupixent, sufferers and suppliers have a brand new choice that’s permitted for this illness and has proven enhancements in sustained illness remission and reductions in itch and oral corticosteroid use in comparison with placebo with a wonderful security profile,” Culton advised Healio.
For extra info:
Donna Culton, MD, PhD, may be reached at dermatology@healio.com.