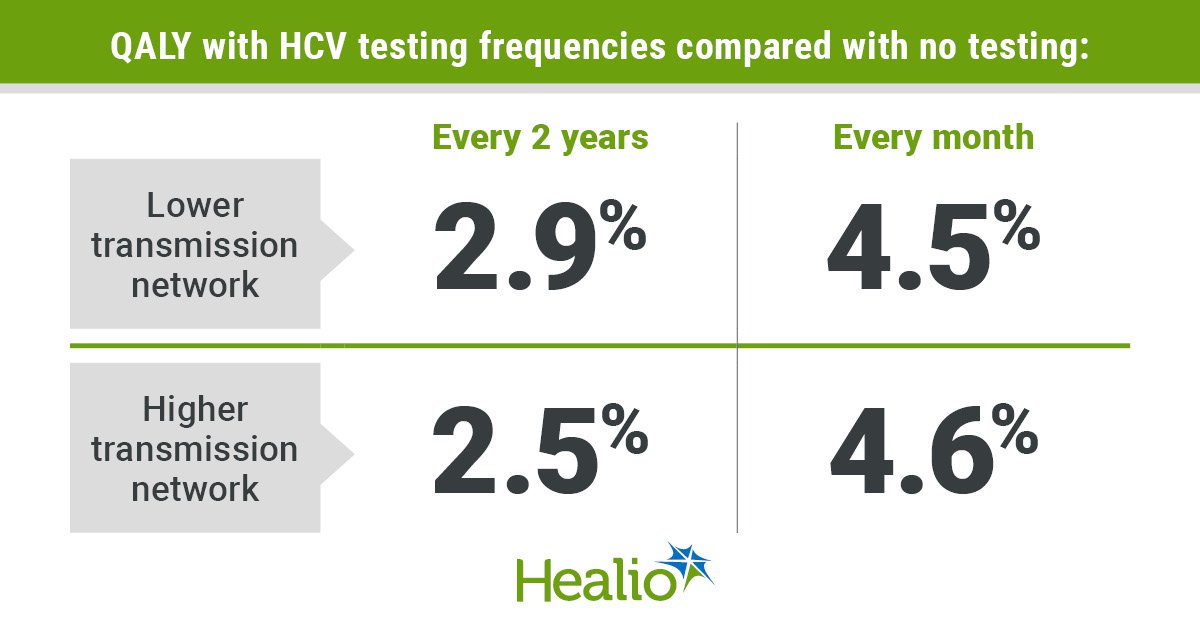
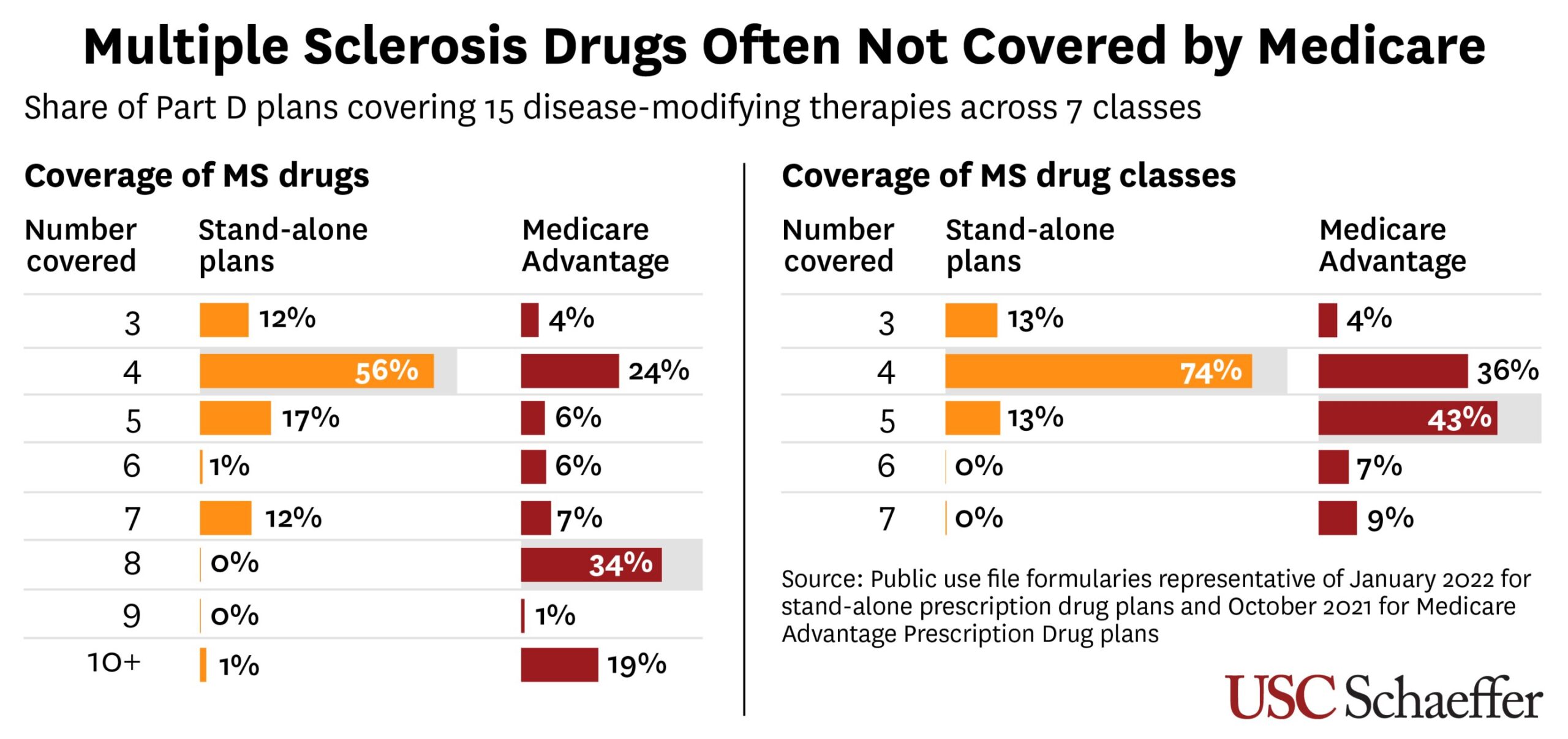
Now that Gilead Sciences has received U.S. regulatory approval for its groundbreaking HIV prevention drug, the corporate has one other hurdle to clear — making certain the drugs could be accessed in low-income international locations the place the illness stays a cussed drawback.
So far as Gilead is anxious, the groundwork exists to satisfy that aim.
Final October, the drugmaker reached voluntary licensing agreements to ultimately make lenacapavir obtainable in 120 largely low- and lower-middle-income international locations. Then, the President’s Emergency Plan for AIDS Aid, or PEPFAR, and the World Fund to Combat AIDS, Tuberculosis, and Malaria crafted an association to cowl dosing for two million folks over three years in quite a few poor international locations.


This text is unique to STAT+ subscribers
Unlock this text — plus in-depth evaluation, newsletters, premium occasions, and information alerts.
Have already got an account? Log in