We’re nonetheless reporting. In case you are a present or former FDA worker or somebody within the trade with details about the company, the protection of generic medicine, or the producers that make them, our staff desires to listen to from you. Megan Rose will be reached on Sign or WhatsApp at 202-805-4865. Debbie Cenziper will be reached on Sign or WhatsApp at 301-222-3133. You too can e-mail us at [email protected].
Reporting Highlights
- Dangerous Drugs: The FDA has given greater than 20 international factories a particular move to proceed sending medicine to the U.S. regardless that they have been made at vegetation that the company had banned.
- Troubled Factories: The medicines got here largely from vegetation in India the place inspectors discovered contaminated medicine, filthy labs and falsified data.
- FDA Secrecy: The company didn’t proactively inform the general public when medicine have been exempted from import bans, and it didn’t routinely check the medicines to make sure they have been secure.
These highlights have been written by the reporters and editors who labored on this story.
On a sweltering morning in western India in 2022, three U.S. inspectors confirmed up unannounced at an enormous pharmaceutical plant surrounded by barricades and barbed wire and demanded to be let inside.
For 2 weeks, they scrutinized buzzing manufacturing traces and laboratories unfold throughout the dense industrial campus, peering over the shoulders of staff on the pill presses, mixers and filling machines that produce dozens of generic medicine for People.
A lot of the manufacturing facility was alleged to be as sterile as an working room. However the inspectors found what seemed to be metallic shavings on drugmaking gear, and data that confirmed vials of medicine that have been “blackish” from contamination had been despatched to america. High quality testing in some instances had been delay for greater than six months, in response to their report, and uncooked supplies tainted with unknown “extraneous matter” have been used anyway, blended into batches of medication.
Solar Pharma’s transgressions have been so egregious that the Meals and Drug Administration imposed one of many authorities’s harshest penalties: banning the manufacturing facility from exporting medicine to america.
However the company, anxious about medicine shortages, instantly undercut its mission to make sure the protection of America’s drug provide.
A secretive group contained in the FDA gave the worldwide producer a particular move to proceed delivery greater than a dozen medicine to america regardless that they have been made on the similar substandard manufacturing facility that the company had formally sanctioned. Drugs and injectable medicines that in any other case would have been banned went to unsuspecting sufferers throughout the nation, together with these with most cancers and epilepsy.
The FDA didn’t routinely check the medicines for high quality issues or use its huge repository of drug-related complaints to proactively observe whether or not they have been harming the individuals who relied on them.
And the company stored the exemptions largely hidden from the general public and from Congress. Even others contained in the FDA have been unaware of the small print.
Within the arms of customers, in response to the FDA’s longtime head of drug security, the knowledge would have triggered “some form of frenzy.”
“We felt we didn’t should make it a public factor,” stated Janet Woodcock, who spent practically 4 a long time on the company.
The exemptions for Solar weren’t a one-time concession. A ProPublica investigation discovered that over a dozen years, the identical small cadre on the FDA granted related exemptions to greater than 20 different factories that had violated crucial requirements in drugmaking, practically all in India. All informed, the group allowed into america at the least 150 medicines or their components from factories with mildew, foul water, soiled labs or fraudulent testing protocols.

Credit score:
Obtained by ProPublica. Highlighted by ProPublica.
A number of the medicine have been recalled — simply earlier than or simply after they have been exempted — due to contaminants or different defects that would trigger well being issues, authorities data present. And a ProPublica evaluation recognized greater than 600 complaints within the FDA’s information about exempted medicine at three of these factories alone, every flagging considerations within the months or years after they have been excluded from import bans in 2022 and 2023.
The “adversarial occasion” experiences about medicine from the Solar plant and two others run by Indian drugmaker Intas Prescription drugs described medicine with an irregular style, odor or residue or sufferers who had skilled sudden or unexplained well being issues.
The experiences cite about 70 hospitalizations and 9 deaths. And people numbers are conservative. ProPublica restricted its depend to experiences that linked issues to a single drug. Nonetheless, the overall variety of complaints to the FDA that point out exempted medicine is within the 1000’s.
“Stomach ache … abdomen was appearing very loopy,” one report stated a few lady utilizing a seizure drug from Solar Pharma. The FDA obtained the criticism in 2023, 9 months after it excluded the medicine from the import ban.
“Feeling actually scorching, breaking out with hives, laborious to breathe, had confusion, glucose stage was excessive, coronary heart charge went up and head, arms and arms bought numb,” famous one other report a few affected person taking a sedative from Intas. The criticism was despatched to the FDA in June 2023, the identical month the company exempted the medicine.
The outcomes described within the complaints might don’t have any connection to the drug or may very well be sudden unwanted side effects. In some instances, the FDA obtained complaints about the identical medicine made by different producers.
Nonetheless, the seriousness of the experiences involving exempted medicine didn’t impress the company to analyze, leaving the general public and the federal government with no method of figuring out whether or not folks have been being harmed and, if that’s the case, what number of.
These unknowns have achieved little to sluggish the exemptions. In 2022, FDA inspectors described a “cascade of failure” at one of many Intas vegetation, discovering staff had destroyed testing data, in a single case pouring acid on some that had been stuffed in a trash bag. On the second Intas manufacturing facility, inspectors stated of their report that data have been “routinely manipulated” to cowl up the presence of particulate matter — which may embody glass, fiber or different contaminants — within the firm’s medicine.

Credit score:
Obtained by ProPublica. Highlighted by ProPublica.
The FDA barred each vegetation in 2023 from delivery medicine to the U.S. Then the company concurrently granted greater than 50 exemptions to these banned factories — the broadest use of exclusions in ProPublica’s evaluation.
Intas, whose U.S. subsidiary is Accord Healthcare, stated in an announcement that the corporate has invested tens of millions of {dollars} in upgrades and new hires and launched a companywide program centered on high quality. Exempted medicine have been despatched to america in a “phased method,” the corporate stated, with third-party oversight and security testing. Intas additionally stated that some exempted medicine have been by no means shipped to america as a result of the FDA discovered different suppliers. The corporate wouldn’t present particulars.
“Intas is effectively on its method in the direction of full remediation of all manufacturing websites,” the corporate stated.
Solar didn’t reply to a number of requests for remark. When the FDA imposed the ban, the corporate stated it might “undertake all obligatory steps to resolve these points and to make sure that the regulator is totally happy with the corporate’s remedial motion. Solar Pharma stays dedicated to being … compliant and in supplying high-quality merchandise to its clients and sufferers globally.”
Each corporations’ factories are nonetheless underneath import bans.
“We’re alleged to have the perfect medication on the earth,” stated Joe DeMayo, a kidney transplant affected person in Philadelphia who took an immunosuppression medicine made by Intas till December 2023, unaware {that a} month earlier the FDA had excused the drug from an import ban. “Why are we shopping for from individuals who aren’t making it proper?”

Credit score:
Hannah Yoon for ProPublica
Sport of Likelihood
How america wound up right here — enjoying a sport of likelihood with dangerous medicine made 1000’s of miles away — is the story of an company that has relentlessly pressed to maintain the availability of low-cost generics flowing at the same time as its personal inspectors warned that a few of these medicine posed a doubtlessly deadly risk to the American public.
The overwhelming majority of the prescriptions stuffed within the nation are for generic medicine, from penicillin to blood thinners to emergency contraception, and lots of of these come from abroad, together with India and China. For years, the FDA has vouched for the standard of generics, assuring the general public in press releases, speeches and social media campaigns that they’re simply as secure and efficient as brand-name medicine.
That assure got here underneath critical query in 2019 when journalist Katherine Eban revealed a breakthrough e book, “Bottle of Lies,” that uncovered rampant fraud and manufacturing violations in Indian factories and the FDA’s reluctance to aggressively examine.
ProPublica recognized one other alarming stage of entrenched failure: Even when the company did examine and single out factories that have been among the many worst in India, it nonetheless gave them entry to American customers. All of the whereas, sufferers took their medication with out query, trusting an company that has lengthy been thought-about the gold customary in drug regulation.
Whereas specialised enterprise publications have generally reported on exemptions after they occur, they’ve provided little context and few specifics.
The FDA in some ways put itself on this untenable place, compelled to resolve between not having sufficient medicine or accepting doubtlessly harmful ones, interviews and authorities data present.
For years, the company gave corporations with a historical past of producing breakdowns approval to provide an more and more bigger share of generic medicine, permitting them to turn into a dominant power in American medication with the ability to disrupt lives if manufacturing traces have been shuttered.
“It’s our personal fault,” stated former FDA inspector Peter Baker, who reported a litany of failures throughout inspections in India and China from 2012 to 2018. “We allowed all these gamers into the market who by no means ought to have been there within the first place. They grew to be monsters and now we will’t return.”
The choices to weaken penalties and permit banned factories to proceed sending medicine to america have been accepted by Woodcock, one of many company’s strongest directors. For greater than 20 years, she led the Middle for Drug Analysis and Analysis, the arm of the FDA that serves because the nation’s gatekeeper for brand spanking new and generic medicine.
In a sequence of interviews with ProPublica, Woodcock stated she supported using exemptions “as a sensible method.”
“We needed to form of cope with the hand we have been dealt,” she stated.

Credit score:
Jason Andrew for ProPublica
Woodcock stated she didn’t see a necessity to tell the general public as a result of the company believed the medicine have been secure. She stated she talked about the follow periodically in closed-door conferences with congressional staffers, however she didn’t present specifics about these conversations.
After Woodcock left her publish in 2020 to assist lead the company’s response to the COVID-19 pandemic, the exemptions — together with these for Solar and Intas — continued underneath her successor, Patrizia Cavazzoni. Cavazzoni, who left the company earlier this yr and rejoined Pfizer, declined to remark.
Former FDA Commissioner Robert Califf, who led the company when Solar and Intas obtained exemptions, informed ProPublica that robust calls needed to be made and the follow didn’t fear him.
The FDA didn’t reply to questions on who made these selections or how the medicine have been evaluated, and it declined requests for interviews with officers who at the moment oversee drug regulation. In an e-mail, the company stated the exemptions are “completely evaluated by means of a multi-disciplinary method.”
Years after the FDA began granting exemptions, some present and former officers say they wrestle with a lingering worry that dangerous medicine are circulating in america.
“It’s not even a hypothetical,” stated one senior FDA worker acquainted with the exemptions, who, like others, spoke on the situation of anonymity as a result of they weren’t licensed to talk publicly. “It’s not a query of if — it’s a query of how a lot.”
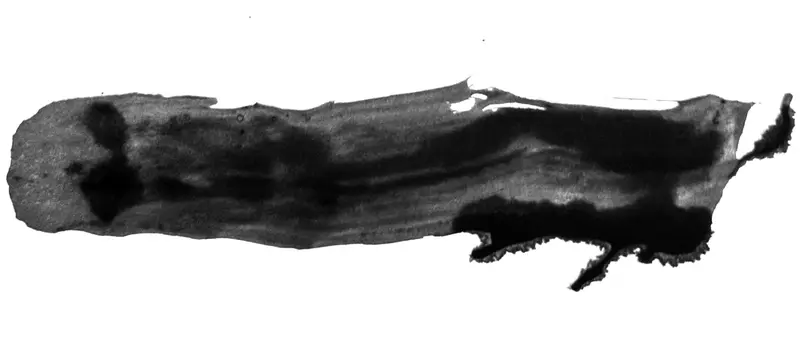
“It Was Rotten Eggs”
Though the FDA has been giving corporations a method round import bans since at the least 2013, the inner course of was so secretive that many present and former FDA officers stated they don’t know what number of exemptions have been granted or for what medicine. In an e-mail, the company stated it didn’t preserve a complete record.
Even two high-level FDA workers members who labored on drug scarcity challenges for the company stated in interviews that they had by no means heard of the exemptions.
Congress required the FDA in 2012 to offer particular data yearly about how and when the company relaxed its guidelines for errant drugmakers to forestall shortages. However the FDA didn’t point out exemptions to import bans till 2024 — and solely then in a single footnote of its 25-page report back to Congress.
ProPublica uncovered the frequent use of exemptions by trying to find the “import alert” record revealed on the FDA’s web site that names factories banned from the U.S. market. As a result of the company publishes solely a present record and doesn’t make the outdated ones public, the information group used web archives and FDA paperwork maintained by the info analytics firm Redica Programs, finally compiling import alerts courting again greater than a decade. The lists establish the medicine exempted from bans however present few different particulars.
ProPublica reviewed scores of inspection experiences and company paperwork for abroad factories and interviewed greater than 200 folks, together with present and former officers of the FDA, to grasp the little-known follow and the continued risk posed by the company’s selections.
The investigation revealed not solely what number of medicine obtained exemptions from import bans, but additionally how lengthy the FDA allowed these exemptions to remain in place — in some instances for years.
The company has eliminated exemptions when there isn’t a longer a scarcity concern. In these instances, the medicine are then banned together with the others on the manufacturing facility. Each Solar and Intas have had medicine that misplaced their exemptions.
Two and a half years after the Solar manufacturing facility was banned, 5 medicine are nonetheless exempted. Intas, whose factories have been banned in 2023, at the moment has 24 medicine on the record. The bans themselves are eliminated solely after corporations repair the issues.
Earlier this month, the FDA went again to the Solar Pharma manufacturing facility for a shock inspection and located ongoing issues, in response to a Solar submitting with the Indian inventory change and Indian media experiences. The considerations centered on the way in which sterile medicine have been made, together with a few of the exempted medicine nonetheless being despatched to america, in response to an individual acquainted with the scenario who didn’t wish to be named as a result of they weren’t licensed to talk publicly.
The FDA stated it put protections in place for exempted medicine: Producers are required to conduct extra high quality checks earlier than they’re despatched to america. That has included further drug-safety testing, in some instances at an impartial lab, and bringing on third-party consultants to confirm the outcomes.
The company didn’t present ProPublica with the names of the third-party consultants employed by Solar and Intas. Intas declined to call its consultants.
“The percentages of those medicine really not being secure or efficient is tiny due to the safeguards,” stated one former FDA official concerned within the exemptions who declined to be named as a result of he nonetheless works within the trade and fears skilled retribution. “Though the power sucks, it’s getting examined extra typically and it’s having impartial eyes on it.”
However present and former FDA inspectors stated these security measures require trusting the vigilance of corporations that have been banned, at the least partially, for offering unreliable or misleading check outcomes to the federal government or failing to analyze experiences about medicine with contaminants or different high quality considerations.

Credit score:
Obtained by ProPublica. Highlighted by ProPublica.
The FDA may have achieved its personal routine testing of the exempted medicine however selected to not. The company stated in an e-mail that it exams the medicine utilizing a “risk-based method” however wouldn’t present ProPublica with any details about which medicine have been examined and what the outcomes have been.
Woodcock stated testing was costly and budgets have been tight. She acknowledged that commonly assessing the exempted medicine for high quality or security considerations “would have enhanced our confidence … and made everybody extra snug.”
The European Union, in contrast, requires medicine made in India and China to be checked for high quality on EU soil. And the U.S. Division of Protection is conducting its personal testing of greater than three dozen generic medicines and has already recognized efficiency and different high quality points.
“For those who don’t know in regards to the high quality of the product, why are you letting it in?” stated Murray Lumpkin, the FDA’s former deputy commissioner for worldwide applications, who left the company in 2014 earlier than many of the exemptions have been granted.
Past the dearth of testing, the FDA didn’t actively search for patterns of hurt among the many exempted medicine in its adversarial occasion database, Woodcock and others stated.
ProPublica’s evaluation of that knowledge discovered 1000’s of experiences each earlier than and after the factories got a move to sidestep import bans. The experiences described sudden instances of cardiac arrest, blurred imaginative and prescient, choking, vertigo and kidney accidents, amongst different points — and in some situations recognized particular considerations about how the medicine have been made.


Credit score:
Obtained by ProPublica
One one who took Intas’ clonazepam, a sedative and epilepsy drug, reported getting “mind zaps” and vivid blue enamel from the coating of dye on the drug. The FDA obtained the criticism the identical month the company exempted the drug from the import ban.
Even earlier than the FDA exempted Intas’ antidepressant bupropion, customers reported that it made them sick, wasn’t all the time efficient and had an irregular odor, which pharmacists and others say can occur when an inactive ingredient breaks down.
“It was rotten eggs,” Nari Miller, a geologist in California who took the drugs in 2022 and had extreme abdomen ache, informed ProPublica. “I opened it and smelled it once I bought house and it was terrible.”
Intas stated it couldn’t reply to particular complaints and that each one medicine have unwanted side effects. “Intas and Accord take note of every adversarial occasion report,” the corporate stated, including, “Accord and Intas are dedicated to persevering with to deliver secure and efficient medicines to sufferers.”
In its assertion, the FDA stated the database is monitored weekly for brand spanking new experiences typically. Woodcock, nevertheless, acknowledged the experiences about exempted medicine, ideally, “could be underneath rather more scrutiny.”
Too Large to Fail
Choices made by the FDA a long time in the past gave rise to using exemptions and the dangers that now confront the American public.
When new brand-name medicine come to market, they’re protected by patents and unique gross sales rights that make them typically costly. When patents expire, generic drug corporations rush in to make their very own variations, that are alleged to be equal to the model. Generics are sometimes far cheaper, and insurance coverage corporations sometimes insist that sufferers use them.
Within the 2000s, as the price of brand-name medicine soared, the FDA started to approve massive numbers of generics. The company, nevertheless, gave a whole lot of these approvals to international producers that had been in hassle earlier than, corporations well-known to the inspectors working to stamp out security and high quality breakdowns at abroad factories, ProPublica discovered.
The FDA granted Solar Pharma alone greater than 250 approvals for generic medicine for the reason that late 2000s, when the corporate began amassing violations, data present. The company’s selections helped to rework the corporate from an area supplier in India to one of many main exporters of medicines to america, with practically $2 billion in annual U.S. gross sales.
The approvals stored coming as inspectors continued to increase considerations about manufacturing practices on the firm’s factories in India, authorities data present.
Extra issues have been discovered at a manufacturing facility that Solar had acquired in Detroit, the place the diabetes drug metformin was contaminated with metallic scrapings. The violations have been so vital that federal marshals in 2009 raided the plant and seized medicine. The corporate finally shuttered the manufacturing facility.
The speedy enlargement of Solar and different international drugmakers set off new alarms amongst inspectors, their supervisors and advisers to Woodcock.
“In a rational system, you’d have stated, ‘This firm just isn’t producing correctly, so let’s not approve any extra of their medicine,” stated William Hubbard, former FDA deputy commissioner for coverage, planning and laws. “The company in a way form of let this occur.”
Ajaz Hussain, the previous deputy director of an FDA workplace that oversaw pharmaceutical science, stated that after leaving the company and turning into a advisor, he made his considerations recognized in conferences with Woodcock and others.
“They’ll’t manufacture it. Why do you retain approving it?” Hussain recalled in an interview with ProPublica. “I stated, ‘Get up.’ … However they didn’t pay attention.”
Hussain in 2012 went to work for Wockhardt, one of many largest pharmaceutical corporations in India, however give up eight months later after he stated he informed his superiors about manufacturing failures within the firm’s factories.
Though FDA inspectors had reported lapses after a number of visits to Wockhardt vegetation between 2004 and 2012, the company cleared the way in which for the corporate to export sedatives, antibiotics, beta blockers, painkillers and different generics to america, data present. Wockhardt obtained exemptions from import bans in 2013. The corporate didn’t reply to repeated requests for remark, however on the time, the corporate stated it was going to rapidly tackle the FDA’s considerations.
The FDA may have denied generic drug purposes — nothing within the legislation prohibits the company from saying no to corporations with spotty observe data. In an e-mail, the FDA stated it considers an organization’s historical past and conducts inspections in some instances earlier than issuing approvals.
Woodcock stated the company knew which factories have been poor performers however feared being sued by corporations blocked from introducing new medicine primarily based on previous conduct. As a substitute, she stated that she tried to persuade drugmakers to spend money on gear and practices that may end up higher-quality medicine.
“We had many conferences about this, and we agonized about all these issues,” she stated.
However little modified.

Shortages vs. High quality
In 2008, dozens of People have been killed by contaminated blood thinner from China. So when Margaret Hamburg was appointed commissioner of the FDA within the aftermath of the disaster, she pressed the company to crack down on abroad drugmakers.
Her efforts ran headlong into what would turn into the worst drug scarcity in trendy historical past. By 2010, most cancers medicine have been scarce. So have been the medicine on hospital crash carts. In all, greater than 200 crucial medicines have been in brief provide.
Razor-thin revenue margins had restricted the variety of corporations that have been prepared to make generic medicine. And the FDA’s enforcement abroad had compelled some manufacturing traces to quickly shut down, which exacerbated the issue.

Credit score:
Brendan Smialowski/Bloomberg Information
Congress lambasted the FDA for the shortages and began requiring the company to show yearly the way it was combatting the issue.
On the time, the FDA had a small staff centered on shortages that operated on the perimeters of Woodcock’s 4,000-person Middle for Drug Analysis and Analysis. With the strain on, Woodcock elevated the staff in 2010 to report on to her deputy, a transfer that gave these workers members a commanding voice on the highest ranges of the company, a number of former staffers informed ProPublica.
After 16 years in prime management roles, Woodcock was formidable sufficient to power a tradition change. Standing 5’2” in FDA convention rooms the place she had typically been disregarded because the lone lady, Woodcock had fought for her standing — generally, she stated, pushed practically to tears with frustration. The board-certified internist asserted her authority by wielding knowledge, what she referred to as “brute power” and the mushy persuasion of an occasional present of an orchid, picked from her backyard in suburban Maryland.

Credit score:
Jason Andrew for ProPublica
By 2010, Woodcock had marshalled the middle right into a powerhouse with nice independence — in some ways, outdoors the attain of the political whims of the commissioners who got here and went. Those that labored along with her through the years stated regardless of her approachable method, she fiercely guarded her territory.
Within the convention room subsequent to Woodcock’s workplace, the drug scarcity workers started to weigh in each time the FDA’s compliance staff moved to penalize wayward drugmakers due to dangerous inspections, in response to a number of former FDA officers concerned within the deliberations.
Typically the small group would resolve {that a} manufacturing facility may now not ship medicine to america and would attempt to get different producers to make extra. And different occasions, the group decided that exemptions from import bans have been the one course.
Discussions may very well be tense and sometimes lasted for weeks. A former worker on the compliance staff informed ProPublica that they repeatedly argued to impose a complete import ban on a international manufacturing facility as a result of they feared the medicine couldn’t be trusted. They have been left feeling uncomfortable about an exemption granted anyway — for a product that they might not use themselves.
With out exemptions, Woodcock informed ProPublica, the FDA might need been compelled to supply the medicine from a “completely unknown producer, say, from China or someplace.”
Present and former FDA officers stated the concessions turned a yearslong follow relatively than a stopgap measure and that the protections put in place by the company weren’t ample. They query why Woodcock and her successor didn’t do extra to lift alarms with Congress or the general public in regards to the determination to depend on insufficient factories for crucial medicine.
Woodcock stated she thought the exemptions have been a symptom of bigger points involving the drug provide that the FDA had no management over — the company, for instance, can’t power corporations involved about slim revenue margins to provide generic medicine.
Two former FDA commissioners informed ProPublica they knew in regards to the follow however weren’t included within the decision-making.
Hamburg, who spent six years on the company underneath the Obama administration, stated the extent of the follow shocked her. “Had I recognized that it was form of an open-ended coverage, I’d have been disturbed,” she stated.
Considered one of her successors, Stephen Hahn, appointed throughout President Donald Trump’s first time period, stated extra folks ought to have been concerned within the selections.
“You’re speaking a few drug of questionable high quality being introduced into the nation,” he stated.
Woodcock stated she didn’t imagine she wanted their enter. “I didn’t assume within the particular person circumstances it was essential to elevate,” she stated, “as a result of what may they do?”
“We Know What Was Discovered”
In 2020, the billionaire founding father of Solar Pharma joined a pivotal convention name with FDA compliance and investigative workers.
Dilip Shanghvi, whose father had run a wholesale drug enterprise in Kolkata, India, began the corporate within the Nineteen Eighties and finally turned Solar Pharma into one of many largest suppliers of generic medicine in america. On the decision, Shanghvi spoke about enhancements at Solar’s huge plant within the Indian metropolis of Halol, in response to an FDA official who attended the assembly.
Amongst different medicine, the plant produced at the least 16 sterile injectables for the U.S. market, in response to a Solar e-mail to the FDA obtained by ProPublica. Injectables are notably harmful if contaminated as a result of the medicine is injected straight into the physique, not like a tablet that goes by means of the filtering of the digestive tract.
In 2018 and 2019, inspectors had reported a sequence of violations on the manufacturing facility, and Solar had obtained greater than 700 complaints about what seemed to be crystals or spider webs forming in considered one of its injectable medicines, data present.
The corporate additionally needed to recall greater than 135,000 vials of vecuronium bromide, a muscle relaxer used throughout surgical procedure, after experiences that the medicine contained glass particles. Solar stated the defect may trigger life-threatening blood clots.
On the decision with the FDA, in response to the company official, Shanghvi assured the federal government that the Halol plant was turning out high-quality merchandise.
But, when the three investigators went again to the manufacturing facility that scorching morning in 2022 for the shock inspection, it was clear inside days that the FDA must take swift motion.
Splitting as much as examine totally different elements of the plant, the inspectors quizzed staff about cleansing procedures and checked out disassembled gear to see if it was contaminated with residue from outdated medicine. At one level, they noticed water leaking close to areas the place sterile medicine have been made, an alarming remark as a result of water can introduce contaminants able to inflicting infections and even demise.
Digging by means of firm data and check outcomes, they discovered extra proof of high quality issues, together with how managers hadn’t correctly investigated a sequence of complaints about international materials, specks, spots and stains in tablets.
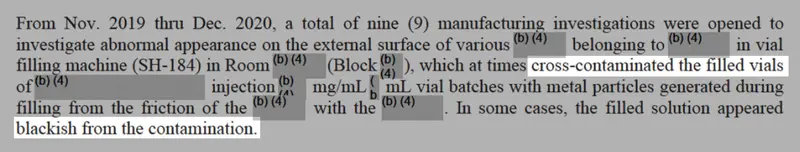
Credit score:
Obtained by ProPublica. Highlighted by ProPublica.
A number of FDA staff acquainted with the inspection report — 23 pages of detailed violations — stated that they had no thought why the company went on to exclude so lots of Solar’s medicine from the following import ban.
“We all know what was discovered,” stated the FDA official who attended the assembly with Shanghvi. “How may you belief [those] medicine?”
Solar didn’t reply to questions in regards to the recollects or its regulatory historical past with the FDA. In its 2023-24 annual report, the corporate stated, “We’ve a relentless concentrate on 24×7 compliance to make sure continuity of provides to our clients and sufferers worldwide.”
The particular findings of the FDA’s newest inspection of the Solar plant performed this month haven’t but been made public, and the corporate didn’t reply to a request for remark.
To some present and former FDA officers and different consultants, plugging a provide scarcity with medicine that could be contaminated or ineffective is not any answer in any respect.
“That may be serving to a scarcity however may be creating a brand new downside,” stated Lumpkin, the previous deputy commissioner.
Final summer season, a pair of FDA investigators arrived at one other manufacturing plant in India that had a bustling manufacturing line. After greater than every week on the Viatris manufacturing facility, they left with a well-known record of security and high quality violations.
The inspectors discovered that gear wasn’t clear and managers did not completely examine unexplained discrepancies in check outcomes.
In an announcement to ProPublica, Viatris stated it instantly labored to resolve the FDA’s considerations. “Affected person security stays our major and unwavering focus,” the corporate stated.
Simply earlier than Christmas, the FDA banned the power from exporting medicine.
Then the company gave the manufacturing facility a move, and 4 of its medicine are nonetheless sure for america.
Patricia Callahan and Vidya Krishnan contributed reporting, and Alice Crites contributed analysis.
Medill Investigative Lab college students Haajrah Gilani, Emma McNamee, Julian Andreone, Isabela Lisco, Aidan Johnstone, Megija Medne, Yiqing Wang, Phillip Powell, Gideon Pardo, Casey He, Lindsey Byman, Josh Sukoff, Kunjal Bastola, Shae Lake, Alyce Brown, Zhiyu Solstice Luo, Jessie Nguyen, Sinyi Au, Kate McQuarrie and Katherine Dailey contributed reporting.
















