Key takeaways:
- The approval permits for rapid therapy following prognosis.
- Sufferers handled in a part 3 research skilled sustained viral response.
Editor’s notice: This can be a creating information story. Please verify again quickly for updates.
FDA authorised a label growth for Mavyret for the therapy of acute hepatitis C virus, in keeping with a press launch from the producer, AbbVie.

The FDA authorised an expanded label for Mavyret to deal with acute hepatitis C.
Mavyret (glecaprevir/pibrentasvir) — first authorised for power HCV in 2017 —is an oral pangenotypic performing antiviral remedy with an 8-week therapy course. The FDA had beforehand granted Mavyret breakthrough remedy designation for acute HCV.
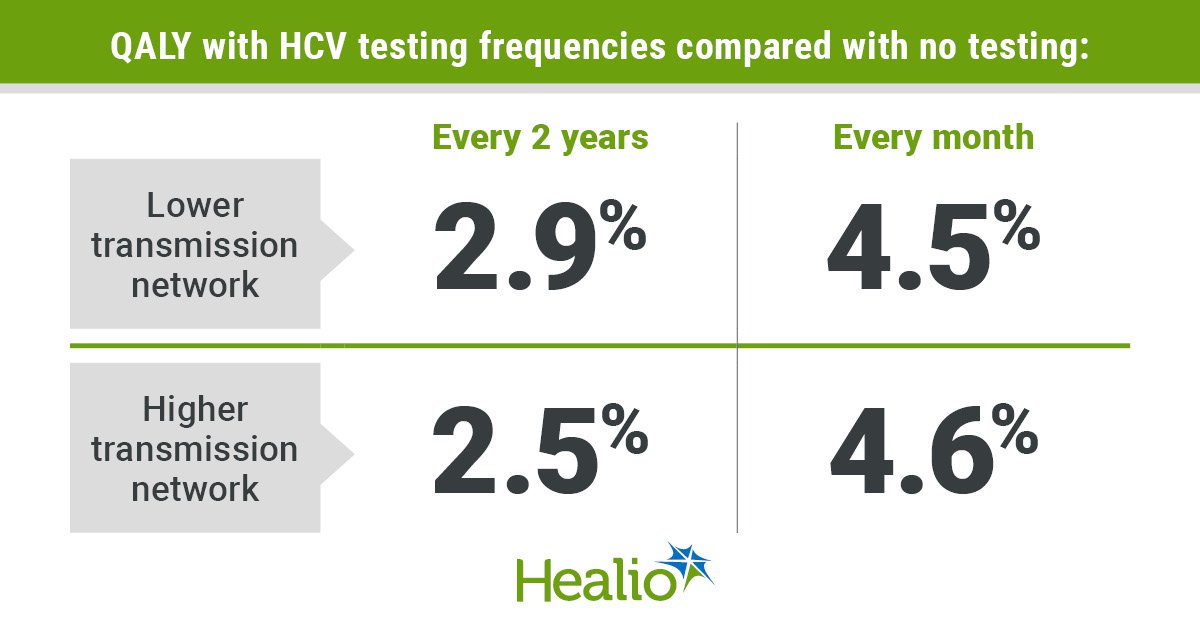
The approval was backed by the outcomes of the part 3 M20-350 scientific trial. The first endpoint of the research was the share of sufferers with sustained virological response 12 weeks after therapy, in keeping with the discharge.
Most adversarial occasions had been gentle or reasonable, in keeping with the discharge.
“Mavyret has handled multiple million sufferers with HCV, however we acknowledge {that a} important want stays for sufferers with acute an infection,” Roopal Thakkar, MD, govt vice chairman for analysis and growth and chief scientific officer at AbbVie, stated within the launch. “The label growth for Mavyret, coupled with the implementation of check and deal with fashions of care, function instruments to assist the general public well being group in treating extra sufferers and bringing us nearer to attaining the worldwide 2030 elimination purpose.”