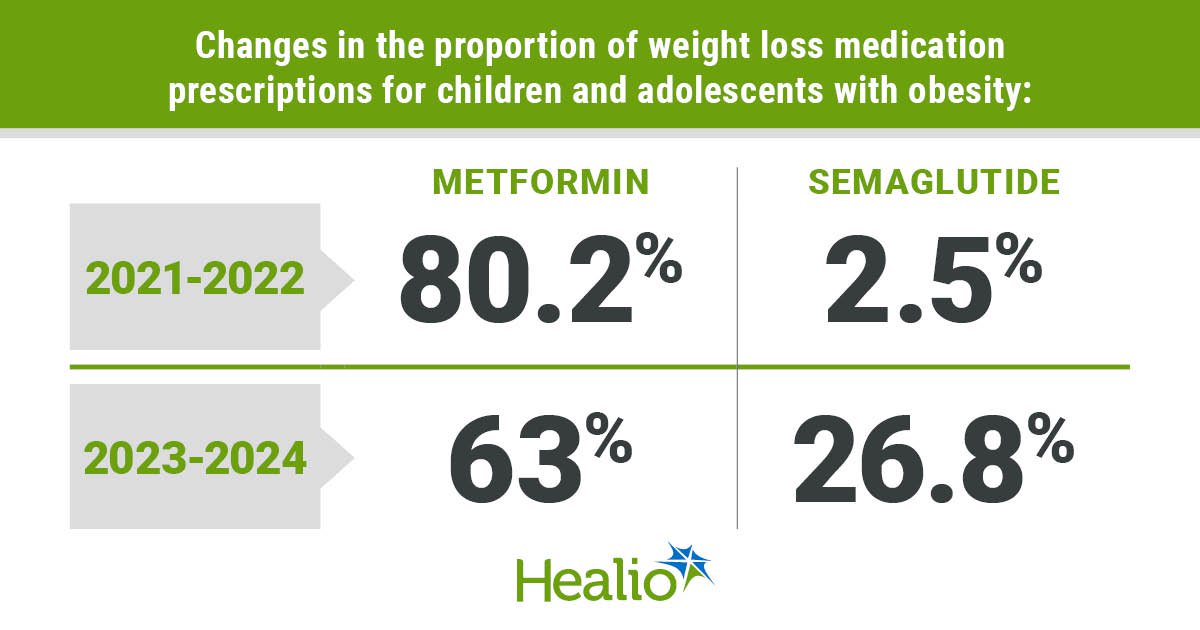
Need to keep on high of the science and politics driving biotech in the present day? Enroll to get our biotech e-newsletter in your inbox.
Good morning, pharma firms are nonetheless struggling to resolve how to reply to Trump’s drug pricing coverage. We focus on in the present day.
The necessity-to-know this morning
- Argenx and Cabaletta Bio, individually, reported new knowledge on potential therapies for myositis, an autoimmune illness that causes muscle weak spot. The info are being offered at a European rheumatology convention.
- RayzeBio, the radiopharmaceutical unit of Bristol Myers Squibb, licensed an experimental drug and diagnostic agent for prostate most cancers from Swiss-based Philochem AG. Beneath phrases of the settlement, Philochem obtained a $350 million upfront fee and is eligible for one more $1 billion in contingency funds.
Drugmakers attempt to strategize with little data on MFN
Within the coming days, the Trump administration is predicted to launch extra particulars on its “most-favored nation” plan aimed toward decreasing drug costs, and pharma firms have been attempting to recreation out attainable situations and strategize how they might reply, my colleague Daniel Payne reviews.
A Washington consultant for one pharmaceutical firm, granted anonymity to debate inner pondering, mentioned the agency expects extra particulars this week, seemingly on Wednesday. The problem is that to date, the present info being shared “is a little bit of a black gap,” the individual mentioned.


This text is unique to STAT+ subscribers
Unlock this text — plus every day protection and evaluation of the biotech sector — by subscribing to STAT+.
Have already got an account? Log in