Key takeaways:
- Sufferers assigned liso-cel achieved longer median PFS and OS in contrast with commonplace of care.
- ORR was considerably increased with liso-cel vs. commonplace of care.
CHICAGO — Lisocabtagene maraleucel considerably improved responses charges and survival outcomes amongst sufferers with relapsed or refractory persistent lymphocytic leukemia, in accordance with findings offered at ASCO Annual Assembly.

William G. Wierda
“Sufferers with CLL or small lymphocytic lymphoma who failed Bruton tyrosine kinase inhibitor and venetoclax have restricted remedy choices and poor prognoses,” in accordance with William G. Wierda, MD, PhD, professor of medication and middle medical director within the division of leukemia at The College of Texas MD Anderson Most cancers Middle, and colleagues. “FDA approval of lisocabtagene maraleucel [Breyanzi, Bristol Myers Squibb] for relapsed or refractory CLL or small lymphocytic lymphoma (SLL) was based mostly on optimistic outcomes from TRANSCEND CLL 004, a single-arm trial.”

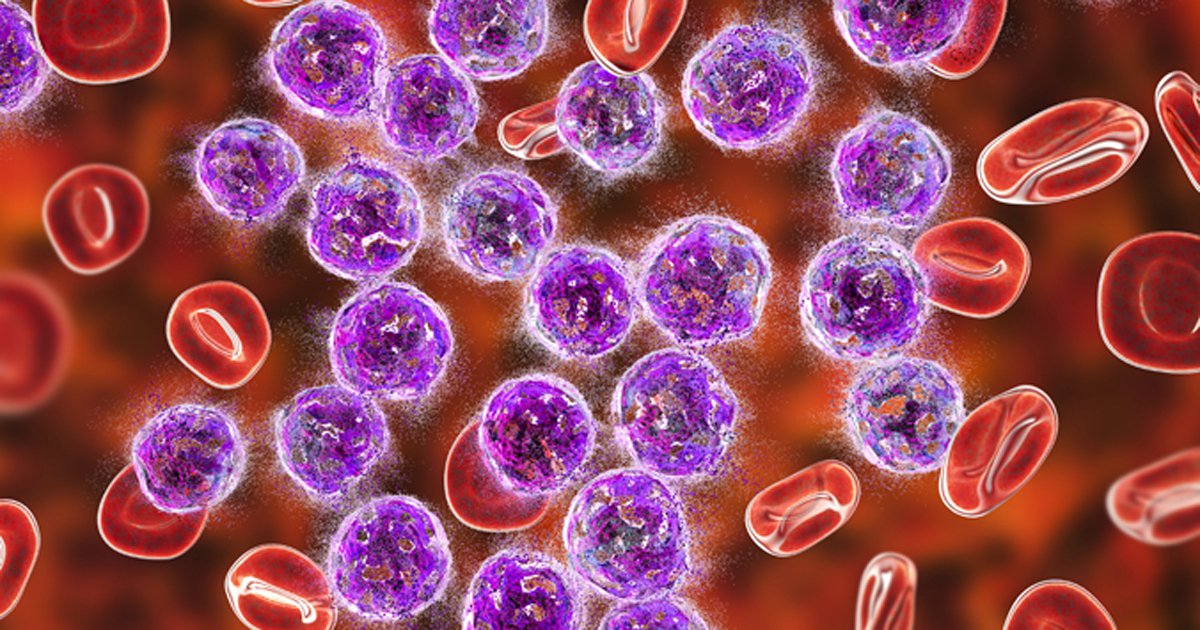
Sufferers with leukemia assigned liso-cel achieved longer median PFS and OS vs. commonplace of care remedy. Picture: Adobe Inventory.
For the present research, Wierda and colleagues sought to evaluate the efficacy of lisocabtagene maraleucel, or liso-cel, amongst 66 sufferers included in TRANSCEND CLL 004 in contrast with commonplace of care amongst an exterior comparator cohort of 212 sufferers included within the Flatiron, COTA and ConcertAI scientific trials.
Commonplace of care therapies included chemotherapy, immunotherapy (excluding chimeric antigen receptor T-cell remedy), Bruton tyrosine kinase inhibitor, venetoclax (Venclexta; AbbVie, Genentech), PI3K inhibitor and mixtures.
Main endpoints included goal response price, PFS and OS.
Median follow-up was 35.4 months with liso-cel and 17.2 months commonplace of care.
Outcomes confirmed an ORR of 52.5% (95% CI, 34.8%-79.2%) with liso-cel vs. 19.2% (95% CI, 14.1%-26.1%) with commonplace of care.
Sufferers within the liso-cel group achieved a median PFS of 12 months (95% CI, 10.8-13.2) in contrast with 4.4 months (95% CI, 3.2-5.5) amongst sufferers who acquired commonplace of care (HR = 0.4; 95% CI, 0.24-0.68). Researchers famous PFS possibilities of 46.3% at 24 months and 30.3% at 36 months with liso-cel in contrast with 11.5% and 5.1%, respectively, with commonplace of care.
Furthermore, median OS was 33.6 months (95% CI, 31.7-35.5) with liso-cel vs. 14.8 months (95% CI, 9.4-20.1) with commonplace of care (HR = 0.47; 95% CI, 0.28-0.79), with OS possibilities of 73.4% at 24 months and 42.6% at 36 months with liso-cel in contrast with 35.1% and 29.7% with commonplace of care.