Key takeaways:
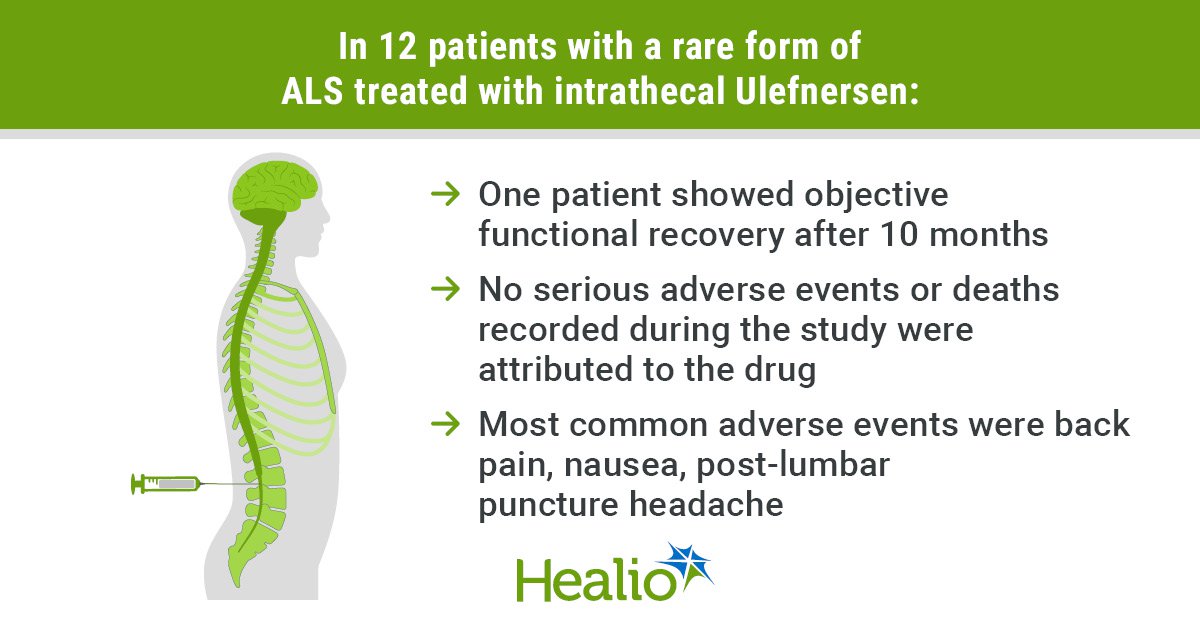
- No critical antagonistic occasions or deaths in the course of the research have been linked to ulefnersen.
- One affected person skilled useful restoration 10 months after remedy.
An antisense oligonucleotide demonstrated a good security profile and its disease-halting impact in a single affected person suggests the drug could also be efficacious in sufferers with a uncommon type of ALS, in accordance with knowledge printed in The Lancet.
“There are medical and pathological similarities between genetic and extra frequent types of ALS, which provides us actual causes to suppose they’ve a standard underlying biology,” Neil A. Shneider, MD, PhD, affiliate professor of neurology at Columbia College Irving Medical Heart and director of the Eleanor and Lou Gehrig ALS Heart, stated in a launch associated to the research.

“By evaluating these ultra-rare sufferers and their response to individualized remedy, we hope to study one thing concerning the biology of the illness that may ultimately assist a bigger share of ALS sufferers,” he stated.
As prior analysis has established that pathogenic variants of fused in sarcoma (FUS) trigger ALS (FUS-ALS), Shneider and colleagues sought to look at the protection and efficacy of ulefnersen (previously jacifusen, Ionis Prescribed drugs), for its potential in arresting the illness course of the uncommon ALS variant.
Their first-in-human, open-label, expanded entry endeavor was carried out throughout 5 websites within the U.S. and Switzerland between June 2019 and June 2023. It included 12 people (median age, 26 years; 58% ladies) who had a medical analysis of ALS, or confirmed medical proof of an FUS variant or motor neuron illness onset.
Contributors have been topic to individualized remedy plans for repeated intrathecal administration of ulefnersen, however common protocol dictated injections have been offered in a number of ascending doses starting from 20 mg to 120 mg over a median length of 9.3 months. The median whole dose was 930 mg, with a median of 9.5 administrations.
On the day of the drug’s administration, researchers assessed motor, neurologic and respiratory operate. Sufferers have been noticed for as much as 24 hours after preliminary administration in addition to following every elevated dose, with statement trimmed to six hours thereafter, adopted by in-person or phone follow-up 3 to 12 days after dosing, with a ultimate phone evaluation 28 days after the ultimate administration.
Focus of neurofilament mild chain (NfL) in cerebrospinal fluid was employed as a biomarker of axonal harm and neurodegeneration, and researchers used the ALS Useful Score Scale-Revised to measure general motor operate. Security outcomes included critical antagonistic occasions and treatment-emergent antagonistic occasions.
Shneider and colleagues reported that 80 treatment-emergent antagonistic occasions have been logged in 11 members, with the most typical being again ache (50%), headache (33%), nausea (25%) and post-lumbar puncture headache (25%).
Moreover, 9 critical antagonistic occasions have been reported in 5 members, primarily regarding respiratory points with none attributed to remedy. Two deaths have been additionally recorded, additionally believed to be non-drug associated. CSF abnormalities have been present in six members, additionally unrelated to remedy.
Though most members confirmed continued useful decline throughout remedy, one affected person skilled what Shneider and colleagues known as “unprecedented, goal useful restoration” after 10 months. One other enrollee remained asymptomatic with documented enchancment in electromyographic abnormalities.
Information additional confirmed NfL focus in CSF was decreased by virtually 83% after 6 months of remedy.
“When testing new medication for ALS, we don’t count on to see medical enchancment,”
Shneider stated within the launch. “What we’ve seen in a single affected person is admittedly unprecedented. It’s shocking and deeply motivating for us, but in addition the neighborhood of ALS sufferers.”
Reference:
Experimental drug might profit some sufferers with uncommon type of ALS. https://www.cuimc.columbia.edu/information/experimental-drug-may-benefit-some-patients-rare-form-als. Revealed Might 22, 2025. Accessed Might 27, 2025.
For extra data:
Neil A. Shneider could be reached at ns327@cumc.columbia.edu.