Key takeaways:
- Mepolizumab decreased the danger for a primary extreme exacerbation and a primary exacerbation requiring ED go to and/or hospitalization.
- The variety of days in intensive care per exacerbation was smaller with this drug.
SAN FRANCISCO — Sufferers with COPD and kind 2 irritation receiving mepolizumab vs. placebo had decreased average and extreme exacerbation charges and fewer ED visits per exacerbation at 104 weeks, in accordance with research findings.
This secondary evaluation of the MATINEE trial, which included sufferers “whatever the presence or absence of power bronchitis or emphysema, and no matter baseline mMRC dyspnea scale grade rating,” was offered on the American Thoracic Society Worldwide Convention.

Knowledge have been derived from Criner GJ, et al. Mepolizumab reduces the danger of extreme exacerbations and healthcare useful resource utilization in power obstructive pulmonary illness: Outcomes from the MATINEE part III randomized managed trial. Introduced at: American Thoracic Society Worldwide Convention; Could 16-21, 2025; San Francisco.

Gerard J. Criner
“Mepolizumab had vital scientific enhancements in the direction of exacerbation discount throughout the broad group of sufferers with COPD who had or didn’t have power bronchitis or proof of emphysema,” Gerard J. Criner, MD, FACP, FACCP, ATSF, chair and professor of thoracic drugs and surgical procedure at Lewis Katz College of Medication at Temple College and director of the Temple Lung Heart, advised Healio.
In a secondary evaluation of the multicenter, double-blind, randomized, placebo-controlled part 3 MATINEE trial, Criner and colleagues evaluated 804 adults aged 40 years or older with COPD and kind 2 irritation (blood eosinophil rely ≥ 300 cells/μL) receiving inhaled triple remedy to find out the impression of subcutaneous 100 mg mepolizumab (Nucala, GSK) vs. placebo each 4 weeks on varied exacerbation outcomes and patient-reported well being care useful resource utilization at week 104.
Included sufferers additionally had at the least two average or one extreme exacerbation up to now 12 months, in accordance with the presentation.
Outcomes from the MATINEE trial at week 52 have been beforehand revealed in The New England Journal of Medication.
Throughout the research inhabitants, 403 sufferers acquired mepolizumab and 401 sufferers acquired placebo.
Researchers noticed that 11% of the mepolizumab group skilled at the least one extreme exacerbation, which was barely lower than the 15% within the placebo group. Moreover, the proportion of sufferers with at the least one extreme exacerbation that required an ED go to and/or hospitalization was smaller amongst sufferers receiving mepolizumab than sufferers receiving placebo (14% vs. 18%).
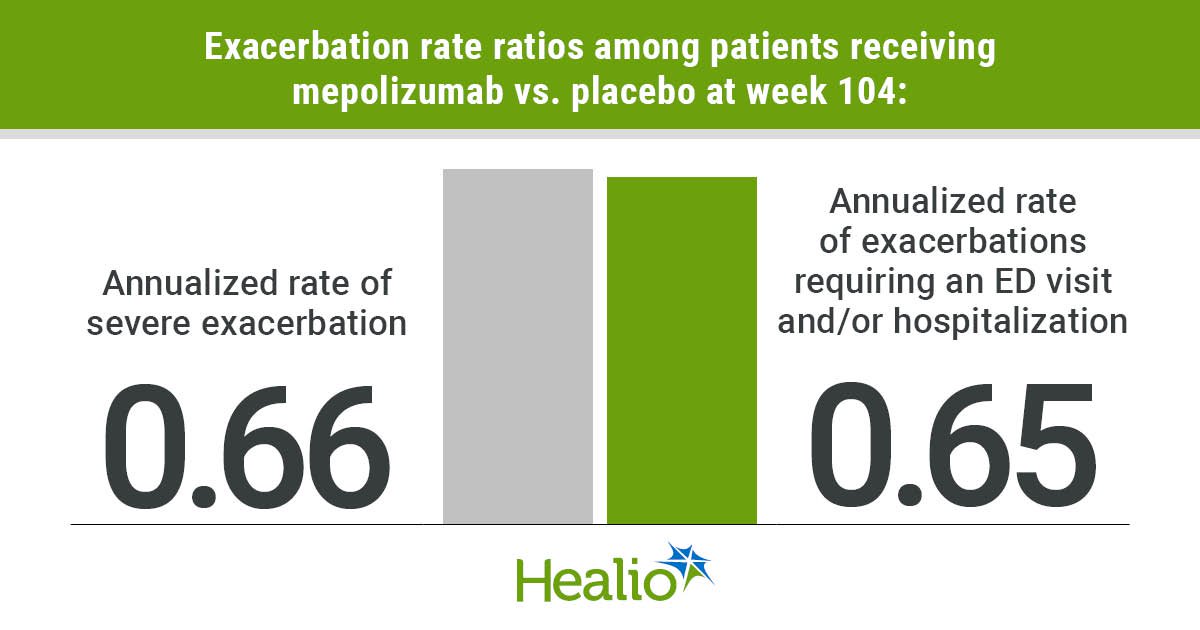
On the 104-week mark, the annualized extreme exacerbation fee fell by 34% (fee ratio [RR] = 0.66; 95% CI, 0.43-1.01) within the mepolizumab vs. placebo group, with 0.1 occasions per yr vs. 0.15 occasions per yr, in accordance with the research.
Equally, there was a discount within the annualized fee of exacerbations requiring an ED go to and/or hospitalization with mepolizumab vs. placebo by 35% (RR = 0.65; 95% CI, 0.43-0.96).
Sufferers receiving mepolizumab had 0.13 occasions per yr, whereas sufferers receiving placebo had 0.2 occasions per yr. Researchers continued to search out higher outcomes associated to exacerbations amongst sufferers receiving mepolizumab vs. placebo throughout evaluation of the danger for a primary extreme exacerbation (HR = 0.74; 95% CI, 0.5-1.08) and the danger for a primary exacerbation that results in an ED go to and/or hospitalization (HR = 0.73; 95% CI, 0.51-1.04).
“Sufferers with COPD and kind 2 irritation can profit from biologic remedy whatever the severity of underlying airflow obstruction with or with out the absence of power bronchitis and emphysema, to scale back average and extreme exacerbations,” Criner advised Healio.
In each the mepolizumab and placebo group, 12% of exacerbations led to basic ward admission. The research additionally reported that 6% of exacerbations in every group led to pressing care/outpatient clinic visits, however the variety of imply visits per exacerbation was barely extra decreased with mepolizumab (0.08 vs. 0.09).
Sufferers receiving mepolizumab vs. placebo had a barely decrease proportion of exacerbations that led to an ED go to (11% vs. 15%) however a barely increased proportion of exacerbations that led to intensive care admissions (5% vs. 4%), in accordance with researchers.
Researchers additional highlighted that the mepolizumab group had a imply variety of 0.14 ED visits per exacerbation, whereas the placebo group had 0.22 visits per exacerbation. Equally, the imply variety of days in intensive care per exacerbation was smaller with mepolizumab vs. placebo (0.17 vs. 0.22).
Criner advised Healio the discovering “that mepolizumab might lower emergency room visits and hospitalization” was very encouraging and “is a major profit for the affected person.”
Trying forward, Criner stated future research will deal with “concentrating on a number of pathways which are key to initiating and sustaining airway and lung irritation in sufferers who’ve COPD, reminiscent of kind 1 and kind 3.”
References:
For extra data:
Gerard J. Criner, MD, FACP, FACCP, ATSF, could be reached at pulmonology@healio.com.