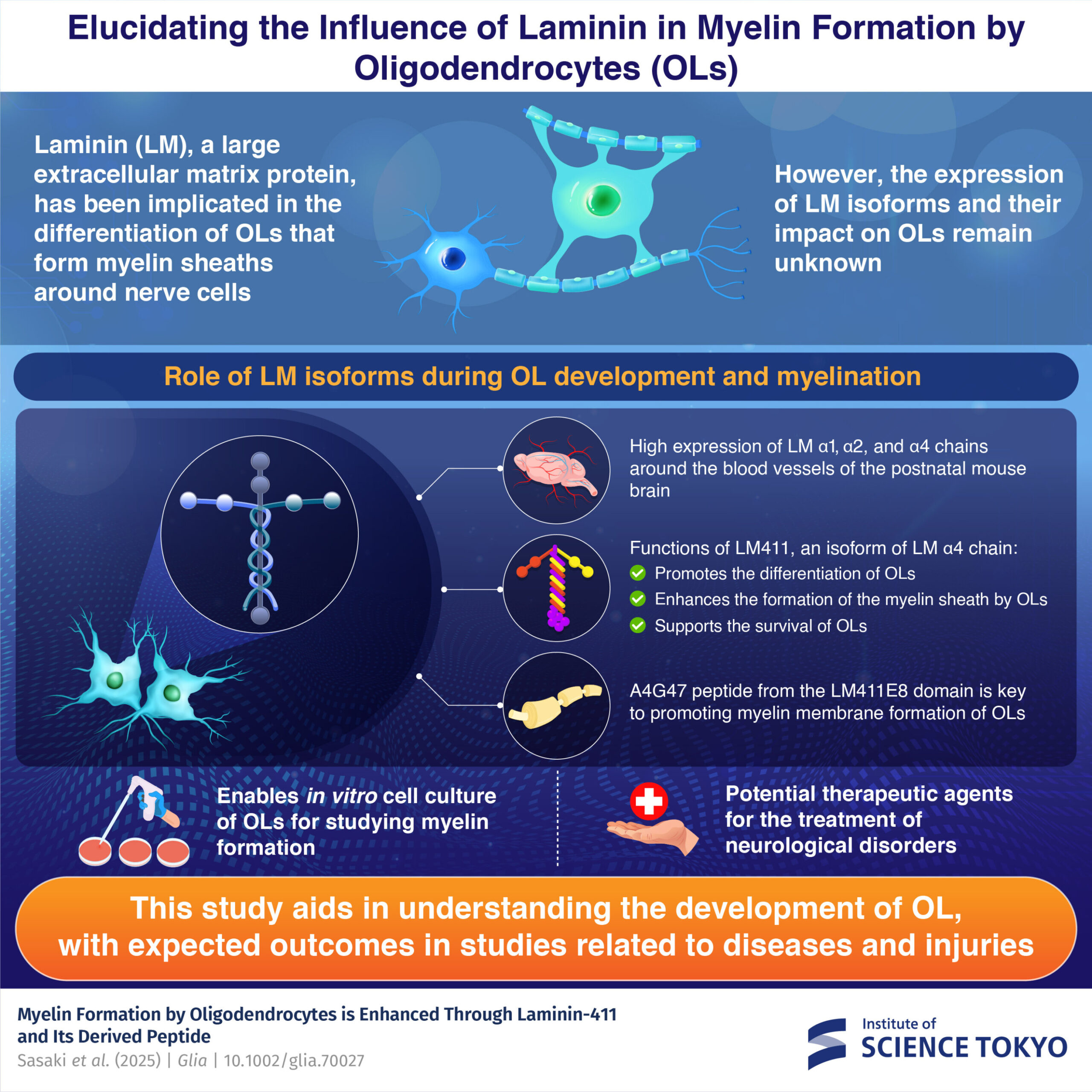
Key takeaways:
- Neuromodulation affords a noninvasive possibility for these with drug-resistant focal epilepsy.
- RNS System led to 62% discount in median % seizure frequency inside 6 months of remedy.
As much as 40% of all epilepsy diagnoses in america are of the drug-resistant selection, affecting 1.2 million folks nationwide.
The RNS System offers a noninvasive remedy possibility for sufferers with this situation, based on a launch from the machine producer.

Healio spoke to Martha Morrell, MD, chief medical officer at NeuroPace and scientific professor of neurology and epileptologist at Stanford College, to search out out extra about its RNS System and the scientific trial that demonstrated its efficacy over 3 years.
Healio: What components led to the choice {that a} machine, slightly than other forms of therapeutics, is likely to be a solution for drug-resistant epilepsy?
Morrell: Regardless of the introduction of many new antiseizure drugs (ASMs) over the previous 15 years, sufferers who don’t reply to trials of two ASMs have lower than a 5% likelihood of attaining seizure management, irrespective of what number of trials of various ASMs observe.
For sufferers with seizures that come up from a discrete area of the mind, akin to focal epilepsy, surgical procedure to take away or ablate the seizure focus can obtain glorious outcomes, even seizure freedom.
Nonetheless, that’s an possibility for under about 15% of sufferers with ASM-resistant focal epilepsy. Neuromodulation is another choice for ASM-resistant focal epilepsy in individuals who aren’t candidates for epilepsy surgical procedure or don’t select to endure a damaging process. The VNS, which stimulates the vagus nerve within the neck, was the primary machine authorized to deal with epilepsy. Subsequently, trials have been performed for units that delivered stimulation on to the mind. One machine is a thalamic obligation cycle stimulator (DBS) and the opposite is the RNS System, the primary direct-brain responsive neuromodulation machine. After feasibility and randomized managed scientific trials, in 2013 the FDA authorized the RNS System to deal with adults with ASM-resistant focal onset epilepsy from one or two seizure foci.
Healio: How and why was the RNS System developed?
Morrell: Discussions a few responsive strategy to deal with epilepsy started within the late Nineteen Nineties. This strategy was imagined as much like cardiac defibrillators that intervene solely when an arrythmia happens. Since epilepsy happens due to an irregular electrical occasion within the mind, and these occasions are rare, this strategy made sense.
Utilizing two leads that each sense and stimulate, containing 4 electrodes every, the RNS System offers stimulation on the location of seizure onset solely in response to irregular electrical exercise.
The doctor identifies the precise irregular electrographic patterns to be detected, in addition to the stimulation parameters. Within the scientific trials, the whole quantity of stimulation delivered was about 3.5 minutes a day. The RNS System neurostimulator shops the placement, date and time of detections of epileptiform exercise, in addition to samples of the intracranial EEG. This information is accessible for the doctor’s evaluation on a safe web-accessed database. The doctor can evaluation this information to trace their affected person’s response to remedy over months and years. This information can also be useful to view adjustments in response to remedy and conduct administration.
Healio: Why was the choice made for the submit–approval research to last as long as 5 years?
Morrell: The post-approval research (PAS) was designed in collaboration with FDA to gather clinically significant details about security and effectiveness in real-world use. Since RNS System is a persistent remedy and because the response to remedy improves with time, the long-term expertise is vital to each doctor and affected person.
Healio: Are you able to talk about the outcomes of the research as offered at AAN and what they portend for these residing with the situation?
Morrell: An open-label, potential PAS was required by FDA as a situation of the RNS System approval. This research was designed to supply real-world information in sufferers handled based on the authorized indication to be used.
Outcomes of the 3-year major effectiveness evaluation and interim security outcomes (the first security evaluation is at 5 years) have been offered on the American Academy of Neurology in April. These information symbolize the biggest multicenter, potential, real-world research of neuromodulation for drug-resistant focal epilepsy thus far.
A complete of 324 sufferers have been enrolled throughout 32 facilities in america. Affected person baseline traits have been much like sufferers collaborating within the earlier feasibility and randomized managed trials.
The effectiveness of RNS System remedy within the post-approval research surpassed the expertise within the early scientific trials. There was a 62% discount within the median % seizure frequency inside 6 months of remedy and that response elevated to 82% at 3 years, with 42% of sufferers experiencing at the least one interval of 6 months or longer with out seizures.
That is notably putting when contemplating that the common length of medically intractable epilepsy was 17.8 years, and the median seizure frequency was six seizures monthly. Effectiveness was related throughout sufferers with one or two seizure onsets and throughout onset location (mesial temporal, neocortical, or each mesial temporal and neocortical).
As within the pre-approval research, remedy was well-tolerated and secure, and the sudden unexplained demise in epilepsy (SUDEP) charge was low. No severe stimulation-related opposed occasions have been reported. Combining information from all RNS System trials (n= 645), the SUDEP charge was 2.3/1000 affected person years; that is considerably decrease than predefined comparators.
Healio: What are the following steps for sufferers and clinicians who use the machine?
Morrell: Because the RNS System has already secured FDA approval, it’s out there at most complete epilepsy facilities all through the U.S.
Sufferers handled within the PAS met the present FDA authorized indication to be used and have been related in baseline traits to sufferers within the earlier trials, together with traits associated to the severity of their focal epilepsy.
Improved seizure outcomes might replicate adjustments in programming practices that come from expertise gained within the preliminary scientific trials, and by studying from the mind information offered by the machine.
Reference:
NeuroPace publicizes information from a long-term post-approval research of the RNS System in focal epilepsy on the 2025 AAN Annual Assembly. https://traders.neuropace.com/news-releases/news-release-details/neuropace-announces-data-long-term-post-approval-study-rns. Printed April 8, 2025. Accessed Could 4, 2025.
For extra info:
Martha Morrell, MD, will be discovered on LinkedIn right here.