Need to keep on high of the science and politics driving biotech immediately? Join to get our biotech publication in your inbox.
Good morning. We’ve obtained extra information out of DC immediately – let’s get into it.
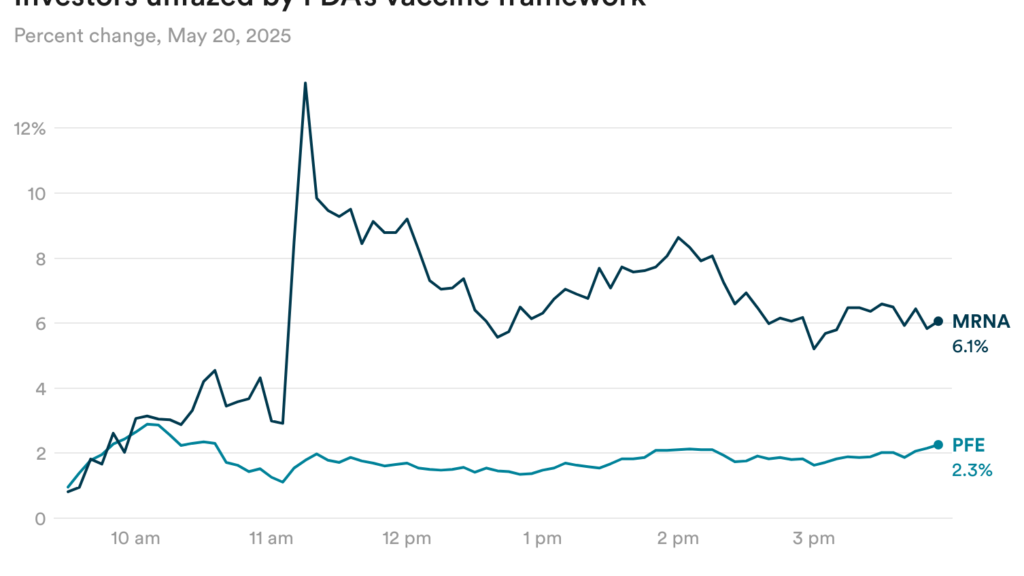
Buyers unfazed by FDA’s Covid vaccine framework
The FDA introduced yesterday that it’s going to restrict entry to Covid-19 vaccines to folks 65 years of age and older and others who’re at excessive danger of turning into critically ailing. It’s going to additionally require producers to conduct medical trials to indicate whether or not the vaccines profit wholesome youthful adults and youngsters.
The framework, revealed within the New England Journal of Drugs, comes after leaders on the company signaled a shift in fascinated with Covid vaccines.
Buyers didn’t appear too involved by the framework, although, as they had been seemingly anticipating a way more restrictive proposal. Shares of Moderna rose 6% yesterday, whereas shares of Pfizer had been up 2% (although Pfizer’s inventory was additionally affected by separate oncology information yesterday.)

In a observe, Jefferies analysts wrote that that the framework gained’t seemingly have an effect on Pfizer’s vaccine gross sales a lot, since many of the income comes from the 65-and-older affected person inhabitants.
In the meantime, TD Cowen analysts wrote that the brand new framework is favorable for Moderna’s next-generation Covid vaccine, which is due for an FDA resolution by the top of this month. Moderna has Part 3 efficacy information for its vaccine that positions it nicely for approval in a broad inhabitants, giving it a aggressive edge over Novavax’s lately authorised vaccine, the analysts wrote.
The framework can be affecting Moderna’s technique with different candidates. The corporate this morning stated it’s withdrawn its FDA utility for a flu/Covid-19 mixture vaccine and plans to resubmit it later this 12 months after it will get efficacy information from an ongoing Part 3 trial. Moderna had already signaled this in earlier steerage.
Some extra particulars on Trump’s drug pricing plan
The Trump administration yesterday shared extra particulars of its plan to push pharmaceutical firms to decrease their U.S. drug costs to be in step with costs in different nations.
Particularly, it’s telling firms to set their U.S. costs on the lowest degree provided in nations which are a part of the Group for Financial Co-operation and Improvement and which have a GDP per capita that’s not less than 60% of the U.S.’ (Nations that seem to satisfy these standards embrace Germany, France, and the U.Okay.)
The pricing targets will apply to brand-name medicine that don’t face competitors from generics or biosimilars. The announcement didn’t embrace a timeline for motion, however stated officers would spotlight commitments by firms to decrease their costs “within the coming weeks.”
There are nonetheless loads of unanswered questions, although, resembling: What are the results for firms that don’t decrease their costs? Which costs (resembling listing or web) are the businesses being requested to decrease?
Learn extra from STAT’s Daniel Payne.
How a lot of an AI drug is definitely made ‘from scratch’?
From my colleague Brittany Trang: Final week, AI drug growth startup Absci introduced that its first candidate, ABS-101 for inflammatory bowel illness, has entered the clinic. Although the corporate doesn’t say so in its press launch, media protection claims that Absci designed the antibody drug “from scratch” with AI.
Earlier this 12 months, we examined these sorts of broad claims made by Absci and one other AI drug growth firm from Flagship referred to as Generate:Biomedicines, and we discovered that they had been enormously exaggerated. At the moment, Absci hadn’t shared many particular particulars about ABS-101.
We now have some extra data. A newly launched patent utility for ABS-101 exhibits that half of the antibody derives from AI.
The place did the remainder of the molecule come from? The corporate didn’t reply to a request from STAT, however Absci CEO Sean McClain has beforehand stated that the corporate studied different TL1A property from Merck and Roche to determine what it needed its mannequin to do.
“I feel it is a nice use case from an IP standpoint — how might we use the AI to get outdoors of the prevailing IP from the rivals however then carve out new IP panorama for our personal, which we now have efficiently carried out with this program?” McClain beforehand stated at a 2024 JPM convention.
A model of this merchandise additionally ran in AI Prognosis, STAT’s subscriber-only publication about AI in well being and drugs written by Brittany Trang. Join right here to get to the most recent developments on AI in well being care and to learn extra evaluation that goals to separate hype from actuality.
Extra reads