Key takeaways:
- Infinant Well being is investigating a dwell biotherapeutic product to forestall necrotizing enterocolitis in preterm infants.
- Trials of an analogous product confirmed a 73% decrease threat for the situation amongst very low-birth-weight infants.
The FDA not too long ago granted orphan drug and uncommon pediatric illness designations to Infinant Well being for a dwell biotherapeutic product to forestall necrotizing enterocolitis in preterm infants, the corporate introduced.
The drug candidate, INF108, features a pressure of Bifidobacterium longum subspecies infantis.

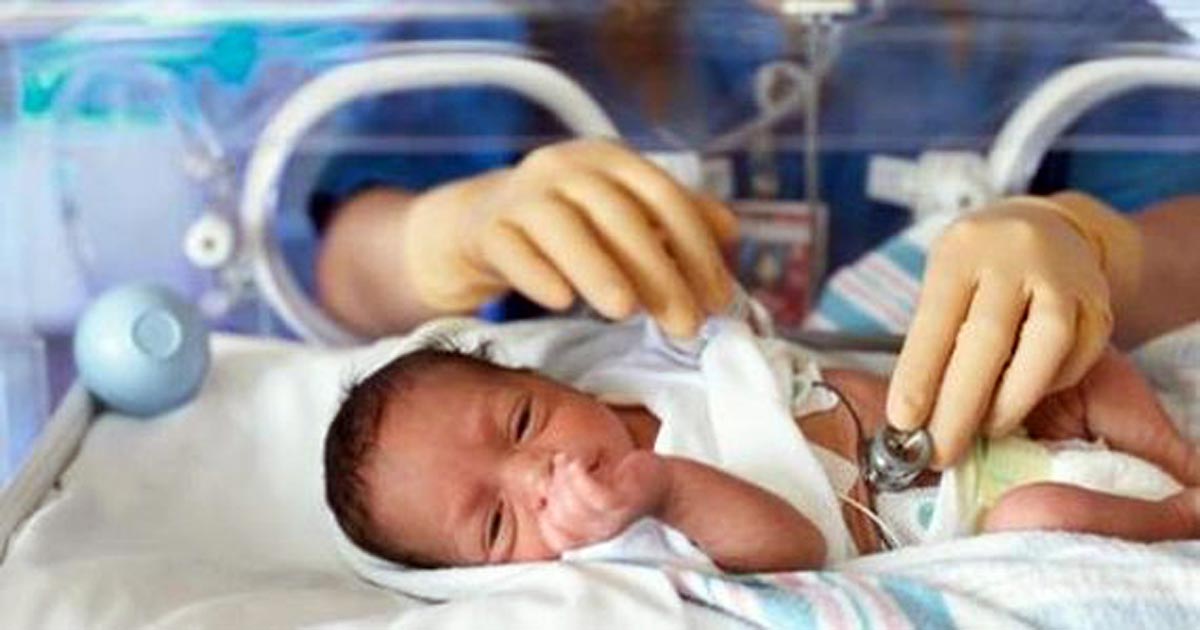
The FDA granted orphan drug designation and uncommon pediatric illness designation to Infinant Well being to develop a dwell biotherapeutic product to forestall necrotizing enterocolitis in preterm infants. Picture: Adobe Inventory.

Arlene Fosmer, MS
“It really works along with breast milk to colonize and defend the gastrointestinal tract of preterm infants, decreasing the degrees of irritation and probably pathogenic micro organism within the toddler’s intestine,” Arlene Fosmer, MS, Infinant Well being’s chief scientific officer, informed Healio.
The corporate utilized for orphan drug designation primarily based on research of an analogous product, EVC001, which is offered underneath the model identify Evivo. In a cohort of 483 infants, EVC001 appeared to cut back the threat for creating necrotizing enterocolitis (NEC) by 73% amongst very-low-birth-weight infants (adjusted threat ratio = 0.27; 95% CI, 0.094-0.614).
Neonatologists beforehand used Bifidobacterium infantis probiotic merchandise off-label to forestall preterm infants from creating NEC, however the FDA instructed physicians to cease doing so in 2023 after an toddler died who was subsequently discovered to have Evivo B. infantis of their blood tradition.

Brian Scottoline, MD, PhD
Brian Scottoline, MD, PhD, professor within the division of neonatology on the Oregon Well being and Science College College of Drugs and a medical adviser aiding with Infinant Well being’s upcoming trials, informed Healio that the FDA’s directions “precipitated a good quantity of concern” amongst neonatologists.
Moreover, he stated the danger for bacteremia or sepsis seems to be very low. A latest systematic evaluate and meta-analysis confirmed that probiotic sepsis occurred in simply eight out of 20,000 infants who obtained probiotics within the NICU, which equates to 1 in each 2,500 infants. The authors identified that for each child who developed probiotic sepsis, 92 infants who didn’t obtain probiotics had been additionally identified with sepsis, “suggesting consideration of risk-benefit could also be warranted.”
“There are a considerable variety of neonatologists who stay very within the potential for B. infantis to stop NEC of their NICUs, pending profitable trial outcomes, security knowledge and approval by the FDA,” Scottoline stated.
Scottoline stated the corporate is taking a number of measures to make sure security throughout upcoming medical trials for INF108, together with testing each batch to confirm that there are not any different organisms, and having clear sepsis detection pointers and protocols within the occasion an toddler does develop a associated an infection.
Fosmer defined that though INF108 is just like EVC001, it is going to be manufactured in an FDA-compliant pharmaceutical facility with processes to make sure the product is as constant and pure as attainable. Due to this, she stated the FDA considers them two distinct merchandise. She additionally famous that EVC001 is a dietary complement for full-term infants and isn’t meant to deal with NEC.
Orphan drug designation waives the FDA’s biologics license software payment, which Fosmer stated will save the corporate greater than $4 million, and pending profitable medical trials, the designation additionally presents the chance for market exclusivity.
Prior to now, the FDA may challenge a precedence evaluate voucher to drug purposes with uncommon pediatric illness designation, however this system expired in December 2024.
“Orphan drug designation and uncommon pediatric illness designation improve investor confidence in our firm, facilitating the fundraising essential to advance our pharmaceutical product growth, together with conducting a part 1 security trial, adopted by a part 2/3 efficacy trial,” Fosmer stated.
Fosmer stated the corporate is planning to launch a part 1 security trial in early 2026.
“We recognize the unwavering assist from advocacy teams just like the NEC Society, affected person households and suppliers throughout the US who’ve been instrumental in supporting this journey and advancing our mission,” Fosmer stated.
References:
For extra data:
Arlene Fosmer, MS, will be reached at afosmer@infinanthealth.com.
Brian Scottoline, MD, PhD, will be reached at scottoli@ohsu.edu.