Key takeaways:
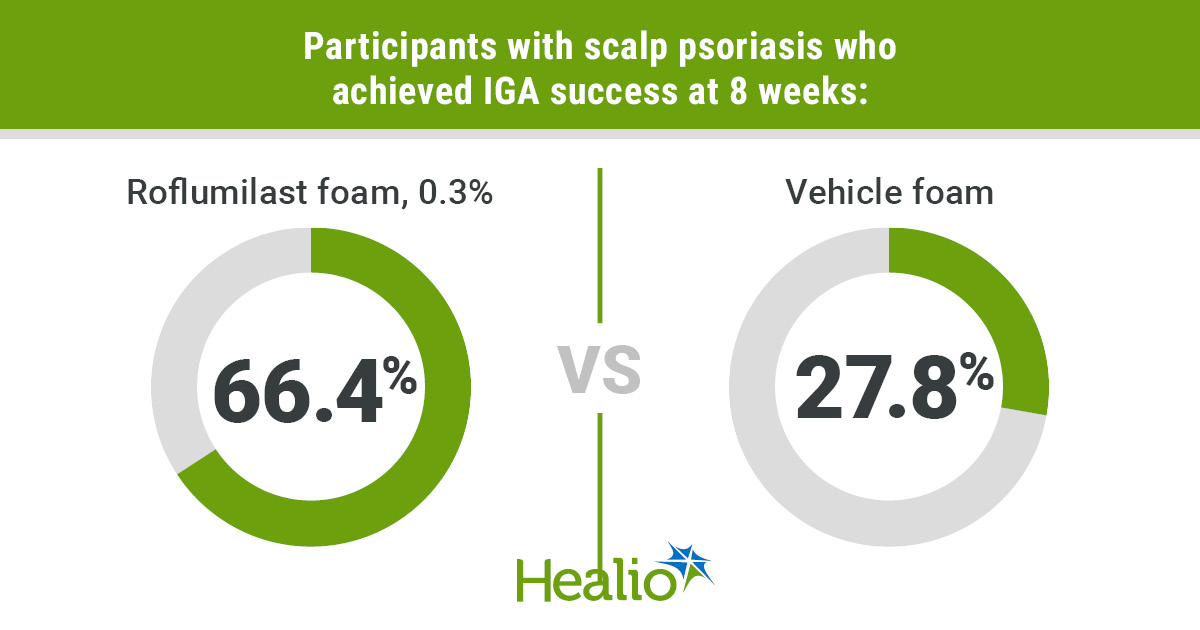
- By week 8, sufferers handled with roflumilast noticed greater efficacy outcomes than these handled with the car.
- Practically all of the treatment-emergent hostile occasions had been gentle to reasonable in severity.
Roflumilast foam, 0.3%, proved to be a simplified remedy routine with excessive efficacy for sufferers with scalp and physique psoriasis, in keeping with a examine revealed in JAMA Dermatology.
In line with Patrick Burnett, MD, PhD, FAAD, chief medical officer at Arcutis and one of many examine investigators, the froth formulation of roflumilast can extra effectively deal with psoriasis-affected areas of the pores and skin with and with out hair, whereas lotions and ointments can’t be used simply.

Knowledge derived from Gooderham MJ, et al. JAMA Dermatol. 2025;doi: 10.1001/jamadermatol.2025.1136.
“When sufferers have scalp involvement, oftentimes their routine will get to be fairly advanced,” Burnett informed Healio. “The concept of a single remedy that can be utilized throughout all of the areas the place a affected person may need psoriasis is exclusive and interesting to well being care suppliers in addition to sufferers.”

Patrick Burnett
Within the section 3 ARRECTOR examine, Burnett and colleagues evaluated the efficacy and security of once-daily roflumilast foam, 0.3% (Zoryve, Arcutis), for the remedy of scalp psoriasis. Researchers randomly assigned contributors to obtain roflumilast (n = 281) or car (n = 151) for 8 weeks.
By week 8, outcomes confirmed that contributors handled with roflumilast noticed greater efficacy outcomes than these handled with the car. Within the roflumilast group, 66.4% of contributors with scalp psoriasis reached IGA success, outlined as an IGA rating of 0 or 1 plus a two-grade enchancment from baseline, in contrast with 27.8% within the car group (P < .001).
Equally, 45.5% of these with physique psoriasis within the roflumilast group additionally achieved IGA success vs. 20.1% within the car group (P < .001).
Within the roflumilast group, 25.2%, 46.2% and 65.3% of contributors achieved Scalp Itch-Numeric Score Scale success, outlined as a minimum of a four-point enchancment from baseline amongst these with a baseline rating of 4 or larger, at weeks 2, 4 and eight, respectively, in contrast with 8%, 16.8% and 30.3% of the car group.
Remedy-emergent hostile occasions occurred in 26.7% of contributors handled with roflumilast vs. 16.6% handled with the car. Of the 75 contributors that skilled treatment-emergent hostile occasions within the roflumilast group, 72 reported the occasions to be gentle or reasonable in severity, whereas two had been severe.
“Essentially the most frequent hostile occasions that we noticed had been headache, diarrhea and nausea,” Burnett stated. “These are issues we have now seen at low frequency throughout all of our improvement packages and can also be one thing that anybody who has used an oral [phosphodiesterase 4] inhibitor is accustomed to and in a position to handle..”
As Healio beforehand reported, FDA accepted Arcutis Biotherapeutics’ supplemental new drug utility for roflumilast foam, 0.3% for the remedy of scalp and physique psoriasis in September. In line with Burnett, the FDA scheduled a Prescription Drug Consumer Price Act date of Might 22, for roflumilast foam, 0.3%. If accepted, roflumilast foam may provide sufferers a “distinctive possibility” that can simplify the remedy strategy of this difficult illness, Burnett informed Healio.
For extra data:
Patrick Burnett, MD, PhD, FAAD, may be reached at dermatology@healio.com.