Wish to keep on prime of the science and politics driving biotech at this time? Enroll to get our biotech publication in your inbox.
Morning! Right this moment, we examine some candid conversations amongst biotech leaders at a Palo Alto convention, see the FDA rehire some laid-off staff, and extra.
One other Cytokinetics personal aim leaves traders fuming
From my colleague Adam Feuerstein: Cytokinetics… argh.
The corporate dedicated an unforced error that has prolonged the evaluate of its coronary heart illness drug by three months, knocked down its inventory worth, and as soon as once more, triggered the ire of traders over questionable administration decision-making.
We discovered yesterday that the FDA desires to evaluate a security administration plan for the Cytokinetics’ drug, referred to as aficamten. The issue: The corporate, inexplicably, by no means submitted one in its authentic software. Now it has, however the FDA wants an additional three months to evaluate it.
Consequently, the anticipated approval determination date for aficamten was pushed again to Dec. 26 from Sept. 26.
Cytokinetics shares are down and investor are indignant.
Biotech business will get candid about politics at Stanford
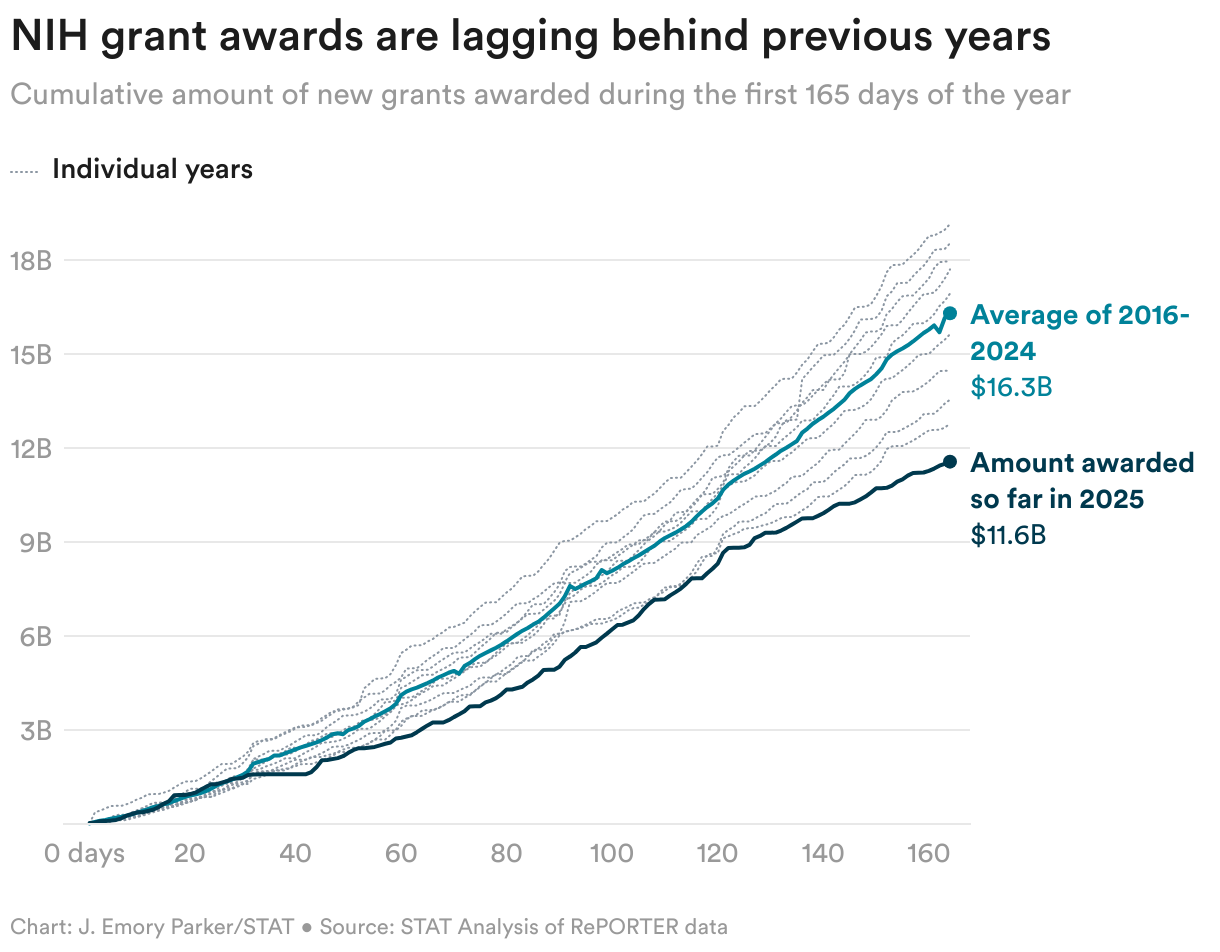
Biotech leaders gathered at Stanford’s Drug Discovery Symposium in Palo Alto this week to voice rising alarm over the Trump administration’s disruption of science — from sweeping FDA layoffs to NIH grant cuts to the hollowing out of variety initiatives. The uncertainty is alerady reshaping R&D methods, STAT’s Jonathan Wosen writes, together with funding choices and medical trial places.
Juxtaposed with the maelstrom that’s AI, the business is uncertain fairly the best way to proceed: “I’ve by no means been extra optimistic on the one hand about our skill to innovate and have impression,” Amgen CEO Robert Bradway stated. “However I’ve by no means been extra involved in regards to the ecosystem by which that innovation happens.”
FDA rehires fired workers amid mounting backlash
In a chaotic about-face, the Meals and Drug Administration is quietly reinstating dozens of laid-off staff — together with journey coordinators, meals scientists, and FOIA workers — after the sweeping spherical of DOGE-initiated layoffs slashed about 20% of its workforce. The abrupt rehiring comes because the company scrambles to satisfy authorized deadlines and preserve core security operations.
Regardless of Commissioner Marty Makary’s repeated claims that no FDA scientists had been affected, no less than two dozen meals security researchers had been let go, solely to be advised they could now return.
“I hope Commissioner Makary continues to evaluate these ill-informed cuts and works to carry again impacted staff expeditiously,” stated Susan Mayne, a former meals director at FDA. “His legacy as commissioner is on the road.”
The impression of Trump’s first 100 days on biotech
On this week’s episode of the Readout LOUD, we evaluate President Trump’s first 100 days, the return of biotech M&A, sturdy drug launches, and Eli Lilly’s PBM downside. The primary 100 days of the Trump administration have had a profound impression on federal well being companies, medical analysis, well being coverage, and the biotech and prescription drugs industries.
To assist us make sense of all of the cuts and coverage shifts, we discuss with STAT editors Gideon Gil and Zach Tracer for his or her perspective overseeing STAT’s reporting on science and politics.
However first, your hosts, Elaine, Allison, and Adam, gab in regards to the week’s comparatively constructive biotech information, together with two acquisitions and a collection of sturdy drug launches. What’s a podcast with out dipping into weight problems, so Elaine will clarify the most recent maneuverings between Eli Lilly and Novo Nordisk round affected person entry and compounding.
Extra reads
- WHO to again use of weight-loss medication for adults globally, raises value subject, Reuters
-
Arvinas lays off 33% of workers, axes Pfizer-partnered part 3 trials after seeing combined knowledge, FierceBiotech
- Amgen says FDA maintain is lifted on its Section 1 weight problems candidate as MariTide enters Section 3, Endpoints