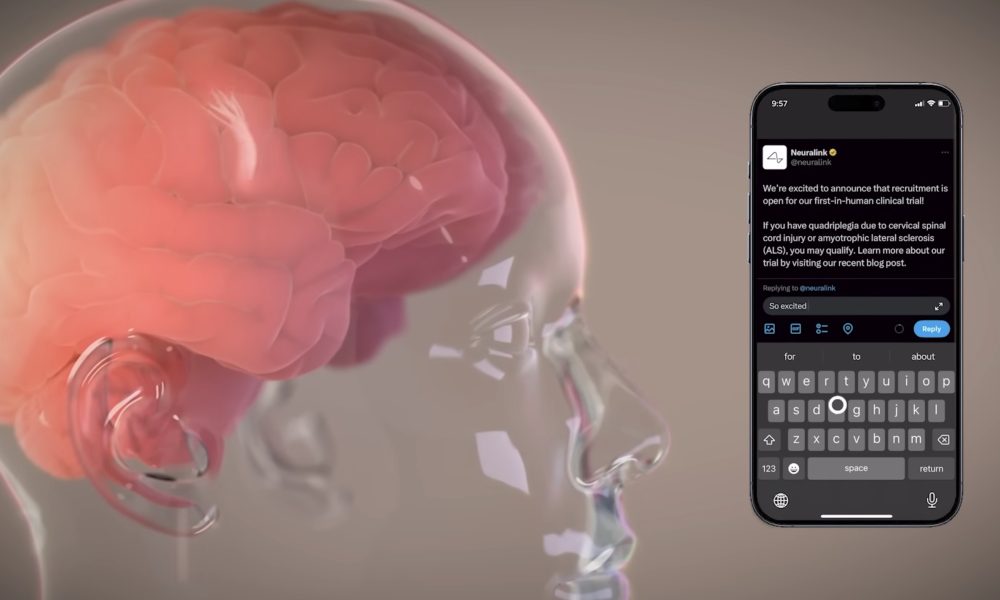
Neuralink’s brain-computer interface (BCI) gadget, Hyperlink, has secured the U.S. Meals and Drug Administration’s (FDA) “breakthrough” designation for restoring communication in sufferers with extreme speech impairment. This milestone advances Elon Musk’s imaginative and prescient of merging human cognition with know-how.
The Hyperlink gadget targets people with neurological situations like Amyotrophic Lateral Sclerosis (ALS), stroke, spinal twine damage, cerebral palsy, and a number of sclerosis. In a latest X video, Neuralink’s third PRIME Examine participant, Bradford G. Smith, who lives with ALS, showcased the gadget’s potential.
Utilizing Hyperlink, Smith regained his skill to speak, leveraging AI to relate with a synthesized model of his former voice. “I’m typing this with my mind,” Smith wrote. “It’s my main communication.”
Smith edited the X video with the assistance of Hyperlink. Within the video, he demonstrated how Hyperlink enabled him to regulate a pc cursor to speak, highlighting the BCI’s skill to interface with exterior gadgets.
Earlier than Hyperlink, Smith relied on a watch tracker, which restricted communication in brilliant settings and restricted his mobility. Now, Neuralink’s implant permits him to attach extra freely. His expertise exhibits Neuralink’s progress in empowering paralyzed people and people with neurodegenerative illnesses by revolutionary assistive options.
The corporate can be exploring functions for imaginative and prescient restoration and different well being challenges. In 2024, Neuralink obtained the FDA’s ‘breakthrough gadget’ tag for its Blindsight gadget. Elon Musk defined that Blindsight would assist individuals who have misplaced each eyes and performance of their optic nerve to see. Nevertheless, Neuralink’s present focus stays on mobility and communication.
Neuralink not too long ago expanded its affected person registry to incorporate individuals worldwide. The PRIME Examine, seemingly the first goal for brand new registrants, exams Hyperlink’s base capabilities. In the meantime, the CONVOY research explores Hyperlink’s skill to regulate robotic gadgets, like an assistive robotic arm. This broader entry underscores Neuralink’s dedication to scaling its trials.
The corporate is reportedly making ready for a $500 million funding spherical, with preliminary talks valuing Neuralink at $8.5 billion pre-money and probably $9 billion post-money, although phrases stay fluid. Neuralink has not commented on the hypothesis about funding.
By incomes FDA breakthrough standing, Neuralink positions Hyperlink as a transformative software for these with extreme speech impairments. Smith’s expertise illustrates its potential to revive independence, whereas ongoing trials and funding efforts sign the corporate’s ambition to redefine human-technology interplay for neurological situations and past.