Key takeaways:
- A transcatheter aortic valve alternative platform was permitted for extreme asymptomatic aortic stenosis.
- Edwards Lifesciences’ TAVR platform is the primary to be permitted on this inhabitants.
Edwards Lifesciences introduced the FDA permitted its transcatheter aortic valve alternative platform for remedy of extreme asymptomatic aortic stenosis, the primary on this affected person inhabitants.
Approval of this platform (SAPIEN 3, SAPIEN 3 Extremely, SAPIEN 3 Extremely RESILIA) was based mostly on the outcomes of the EARLY TAVR trial which have been introduced at TCT 2024 and concurrently printed in The New England Journal of Medication. The trial was designed to judge TAVR in contrast with watchful ready for sufferers with extreme asymptomatic aortic stenosis.

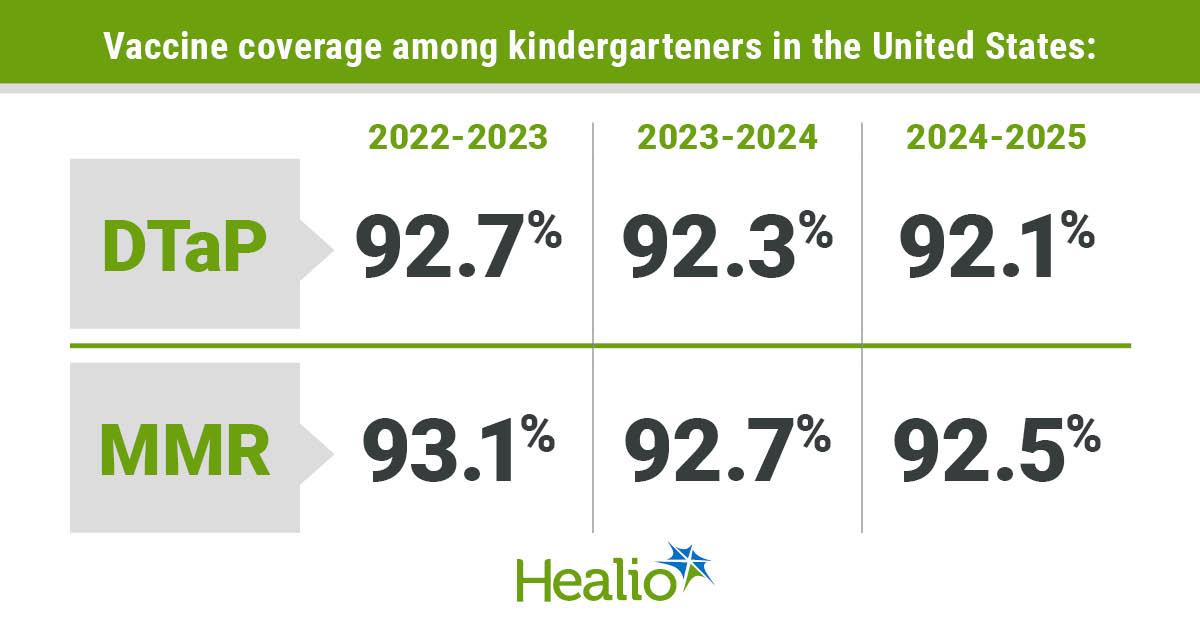
As Healio beforehand reported, over a median follow-up of practically 4 years, 26.8% of sufferers with extreme asymptomatic aortic stenosis assigned to TAVR skilled dying, stroke or unplanned CV hospitalization, in contrast with 45.3% of sufferers assigned to watchful ready.

Philippe Généreux
“There may be an pressing want to vary follow and TAVR pointers for the remedy of aortic stenosis sufferers, which presently suggest ‘watchful ready’ till signs develop,” Philippe Généreux, MD, director of the structural coronary heart program at Gagnon Cardiovascular Institute, Morristown Medical Middle, stated in a press launch. “As we noticed within the EARLY TAVR trial, sufferers initially designated as asymptomatic grew to become symptomatic in sudden and unpredictable methods, underscoring the significance of early analysis by a coronary heart crew to enhance affected person outcomes and profit the healthcare system.”
Editor’s notice: This can be a growing information story. Please test again quickly for updates.